Abstract
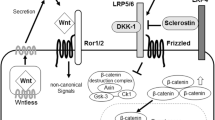
Sclerostin, encoded by the SOST gene, works as an inhibitor of the Wnt pathway and therefore is an important regulator of bone homeostasis. Due to its potent action as an inhibitor of bone formation, blocking sclerostin activity is the purpose of recently developed anti-osteoporotic treatments. Two bone-specific transcription factors, RUNX2 and OSX, have been shown to interact and co-ordinately regulate the expression of bone-specific genes. Although it has been recently shown that sclerostin is targeted by OSX in mice, there is currently no information of whether this is also the case in human cells. We have identified SP-protein family and AML1 consensus binding sequences at the human SOST promoter and have shown that OSX, together with RUNX2, binds to a specific region close to the transcription start site. Furthermore, we show that OSX and RUNX2 activate SOST expression in a co-ordinated manner in vitro and that SOST expression levels show a significant positive correlation with OSX/RUNX2 expression levels in human bone. We also confirmed previous results showing an association of several SOST/RUNX2 polymorphisms with bone mineral density.


Similar content being viewed by others
References
Beighton P (1988) Sclerosteosis. J Med Genet 25:200–203
Beighton P, Barnard A, Hamersma H, van der Wouden A (1984) The syndromic status of sclerosteosis and van Buchem disease. Clin Genet 25:175–181
Balemans W, Ebeling M, Patel N, Van Hul E, Olson P, Dioszegi M, Lacza C, Wuyts W, Van Den Ende J, Willems P, Paes-Alves AF, Hill S, Bueno M, Ramos FJ, Tacconi P, Dikkers FG, Stratakis C, Lindpaintner K, Vickery B, Foernzler D, Van Hul W (2001) Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST). Hum Mol Genet 10:537–543
Brunkow ME, Gardner JC, Van Ness J, Paeper BW, Kovacevich BR, Proll S, Skonier JE, Zhao L, Sabo PJ, Fu Y, Alisch RS, Gillett L, Colbert T, Tacconi P, Galas D, Hamersma H, Beighton P, Mulligan J (2001) Bone dysplasia sclerosteosis results from loss of the SOST gene product, a novel cystine knot-containing protein. Am J Hum Genet 68:577–589
Collette NM, Genetos DC, Economides AN, Xie L, Shahnazari M, Yao W, Lane NE, Harland RM, Loots GG (2012) Targeted deletion of Sost distal enhancer increases bone formation and bone mass. Proc Natl Acad Sci USA 109:14092–14097
van Bezooijen RL, Bronckers AL, Gortzak RA, Hogendoorn PC, van der Wee-Pals L, Balemans W, Oostenbroek HJ, Van Hul W, Hamersma H, Dikkers FG, Hamdy NA, Papapoulos SE, Lowik CW (2009) Sclerostin in mineralized matrices and van Buchem disease. J Dent Res 88:569–574
Semenov M, Tamai K, He X (2005) SOST is a ligand for LRP5/LRP6 and a Wnt signaling inhibitor. J Biol Chem 280:26770–26775
Sevetson B, Taylor S, Pan Y (2004) Cbfa1/RUNX2 directs specific expression of the sclerosteosis gene (SOST). J Biol Chem 279:13849–13858
Yang F, Tang W, So S, de Crombrugghe B, Zhang C (2010) Sclerostin is a direct target of osteoblast-specific transcription factor osterix. Biochem Biophys Res Commun 400:684–688
Ortuno MJ, Susperregui AR, Artigas N, Rosa JL, Ventura F (2013) Osterix induces Col1a1 gene expression through binding to Sp1 sites in the bone enhancer and proximal promoter regions. Bone 52:548–556
Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, de Crombrugghe B (2002) The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell 108:17–29
Doecke JD, Day CJ, Stephens AS, Carter SL, van Daal A, Kotowicz MA, Nicholson GC, Morrison NA (2006) Association of functionally different RUNX2 P2 promoter alleles with BMD. J Bone Miner Res 21:265–273
Ermakov S, Malkin I, Keter M, Kobyliansky E, Livshits G (2008) Family-based association study of polymorphisms in the RUNX2 locus with hand bone length and hand BMD. Ann Hum Genet 72:510–518
Pineda B, Hermenegildo C, Laporta P, Tarin JJ, Cano A, Garcia-Perez MA (2010) Common polymorphisms rather than rare genetic variants of the Runx2 gene are associated with femoral neck BMD in Spanish women. J Bone Miner Metab 28:696–705
Valero C, Zarrabeitia MT, Hernandez JL, Pineda B, Cano A, Garcia-Perez MA, Riancho JA (2011) Relationship of sclerostin and secreted frizzled protein polymorphisms with bone mineral density: an association study with replication in postmenopausal women. Menopause 18:802–807
Timpson NJ, Tobias JH, Richards JB, Soranzo N, Duncan EL, Sims AM, Whittaker P, Kumanduri V, Zhai G, Glaser B, Eisman J, Jones G, Nicholson G, Prince R, Seeman E, Spector TD, Brown MA, Peltonen L, Smith GD, Deloukas P, Evans DM (2009) Common variants in the region around Osterix are associated with bone mineral density and growth in childhood. Hum Mol Genet 18:1510–1517
Estrada K, Styrkarsdottir U, Evangelou E, Hsu YH, Duncan EL, Ntzani EE, Oei L, Albagha OM, Amin N, Kemp JP, Koller DL, Li G, Liu CT, Minster RL, Moayyeri A, Vandenput L, Willner D, Xiao SM, Yerges-Armstrong LM, Zheng HF, Alonso N, Eriksson J, Kammerer CM, Kaptoge SK, Leo PJ, Thorleifsson G, Wilson SG, Wilson JF, Aalto V, Alen M, Aragaki AK, Aspelund T, Center JR, Dailiana Z, Duggan DJ, Garcia M, Garcia-Giralt N, Giroux S, Hallmans G, Hocking LJ, Husted LB, Jameson KA, Khusainova R, Kim GS, Kooperberg C, Koromila T, Kruk M, Laaksonen M, Lacroix AZ, Lee SH, Leung PC, Lewis JR, Masi L, Mencej-Bedrac S, Nguyen TV, Nogues X, Patel MS, Prezelj J, Rose LM, Scollen S, Siggeirsdottir K, Smith AV, Svensson O, Trompet S, Trummer O, van Schoor NM, Woo J, Zhu K, Balcells S, Brandi ML, Buckley BM, Cheng S, Christiansen C, Cooper C, Dedoussis G, Ford I, Frost M, Goltzman D, Gonzalez-Macias J, Kahonen M, Karlsson M, Khusnutdinova E, Koh JM, Kollia P, Langdahl BL, Leslie WD, Lips P, Ljunggren O, Lorenc RS, Marc J, Mellstrom D, Obermayer-Pietsch B, Olmos JM, Pettersson-Kymmer U, Reid DM, Riancho JA, Ridker PM, Rousseau F, Slagboom PE, Tang NL, Urreizti R, Van Hul W, Viikari J, Zarrabeitia MT, Aulchenko YS, Castano-Betancourt M, Grundberg E, Herrera L, Ingvarsson T, Johannsdottir H, Kwan T, Li R, Luben R, Medina-Gomez C, Palsson ST, Reppe S, Rotter JI, Sigurdsson G, van Meurs JB, Verlaan D, Williams FM, Wood AR, Zhou Y, Gautvik KM, Pastinen T, Raychaudhuri S, Cauley JA, Chasman DI, Clark GR, Cummings SR, Danoy P, Dennison EM, Eastell R, Eisman JA, Gudnason V, Hofman A, Jackson RD, Jones G, Jukema JW, Khaw KT, Lehtimaki T, Liu Y, Lorentzon M, McCloskey E, Mitchell BD, Nandakumar K, Nicholson GC, Oostra BA, Peacock M, Pols HA, Prince RL, Raitakari O, Reid IR, Robbins J, Sambrook PN, Sham PC, Shuldiner AR, Tylavsky FA, van Duijn CM, Wareham NJ, Cupples LA, Econs MJ, Evans DM, Harris TB, Kung AW, Psaty BM, Reeve J, Spector TD, Streeten EA, Zillikens MC, Thorsteinsdottir U, Ohlsson C, Karasik D, Richards JB, Brown MA, Stefansson K, Uitterlinden AG, Ralston SH, Ioannidis JP, Kiel DP, Rivadeneira F (2012) Genome-wide meta-analysis identifies 56 bone mineral density loci and reveals 14 loci associated with risk of fracture. Nat Genet 44:491–501
Perez-Campo FM, Sanudo C, Delgado-Calle J, Arozamena J, Zarrabeitia MT, Riancho JA (2014) A Sclerostin super-producer cell line derived from the human cell line SaOS-2: a new tool for the study of the molecular mechanisms driving Sclerostin expression. Calcif Tissue Int 95:194–199
Delgado-Calle J, Sanudo C, Bolado A, Fernandez AF, Arozamena J, Pascual-Carra MA, Rodriguez-Rey JC, Fraga MF, Bonewald L, Riancho JA (2012) DNA methylation contributes to the regulation of sclerostin expression in human osteocytes. J Bone Miner Res 27:926–937
Zhao J, Bradfield JP, Li M, Zhang H, Mentch FD, Wang K, Sleiman PM, Kim CE, Glessner JT, Frackelton EC, Chiavacci RM, Berkowitz RI, Zemel BS, Hakonarson H, Grant SF (2011) BMD-associated variation at the Osterix locus is correlated with childhood obesity in females. Obesity (Silver Spring) 19:1311–1314
Melnikova IN, Crute BE, Wang S, Speck NA (1993) Sequence specificity of the core-binding factor. J Virol 67:2408–2411
Ogawa T, Oda N, Nakashima K, Sasaki H, Hattori M, Sakaki Y, Kihara H, Ohno M (1992) Unusually high conservation of untranslated sequences in cDNAs for Trimeresurus flavoviridis phospholipase A2 isozymes. Proc Natl Acad Sci USA 89:8557–8561
Artigas N, Urena C, Rodriguez-Carballo E, Rosa JL, Ventura F (2014) Mitogen-activated protein kinase (MAPK)-regulated interactions between Osterix and Runx2 are critical for the transcriptional osteogenic program. J Biol Chem 289:27105–27117
Rashid H, Ma C, Chen H, Wang H, Hassan MQ, Sinha K, de Crombrugghe B, Javed A (2014) Sp7 and Runx2 molecular complex synergistically regulate expression of target genes. Connect Tissue Res 55(Suppl 1):83–87
Banerjee C, Javed A, Choi JY, Green J, Rosen V, van Wijnen AJ, Stein JL, Lian JB, Stein GS (2001) Differential regulation of the two principal Runx2/Cbfa1 n-terminal isoforms in response to bone morphogenetic protein-2 during development of the osteoblast phenotype. Endocrinology 142:4026–4039
Bustamante M, Nogues X, Agueda L, Jurado S, Wesselius A, Caceres E, Carreras R, Ciria M, Mellibovsky L, Balcells S, Diez-Perez A, Grinberg D (2007) Promoter 2–1025 T/C polymorphism in the RUNX2 gene is associated with femoral neck bmd in Spanish postmenopausal women. Calcif Tissue Int 81:327–332
Lee HJ, Koh JM, Hwang JY, Choi KY, Lee SH, Park EK, Kim TH, Han BG, Kim GS, Kim SY, Lee JY (2009) Association of a RUNX2 promoter polymorphism with bone mineral density in postmenopausal Korean women. Calcif Tissue Int 84:439–445
Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G (1997) Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell 89:747–754
Komori T (2010) Regulation of osteoblast differentiation by Runx2. Adv Exp Med Biol 658:43–49
St John HC, Hansen SJ, Pike JW (2015) Analysis of SOST expression using large minigenes reveals the MEF2C binding site in the evolutionarily conserved region (ECR5) enhancer mediates forskolin, but not 1,25-dihydroxyvitamin D or TGFbeta responsiveness. J Steroid Biochem Mol Biol. doi:10.1016/j.jsbmb.2015.09.005
Wijenayaka AR, Yang D, Prideaux M, Ito N, Kogawa M, Anderson PH, Morris HA, Solomon LB, Loots GG, Findlay DM, Atkins GJ (2015) 1alpha,25-dihydroxyvitamin D3 stimulates human SOST gene expression and sclerostin secretion. Mol Cell Endocrinol 413:157–167
Leupin O, Kramer I, Collette NM, Loots GG, Natt F, Kneissel M, Keller H (2007) Control of the SOST bone enhancer by PTH using MEF2 transcription factors. J Bone Miner Res 22:1957–1967
Acknowledgments
This research was supported by grants from the Spanish Ministry of Health and the Instituto de Salud Carlos III (PI12-0615), which may be cofunded by the FEDER funds from the European Union, and the Fundación Española de Investigación Osea y Metabolismo Mineral (FEIOMM). C. Sañudo is supported by IDIVAL (Instituto de Investigación Marqués de Valdecilla).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Flor M. Pérez-Campo, Ana Santurtún, Carmen García-Ibarbia, María A. Pascual, Carmen Valero, Carlos Garcés, Carolina Sañudo, María T. Zarrabeitia and José A. Riancho declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
As part of a project to determine genetic factors associated with osteoporosis, the study was approved by the IRB (Comité de Etica en Investigación Clínica de Cantabria) and participants provided informed consent.
Rights and permissions
About this article
Cite this article
Pérez-Campo, F.M., Santurtún, A., García-Ibarbia, C. et al. Osterix and RUNX2 are Transcriptional Regulators of Sclerostin in Human Bone. Calcif Tissue Int 99, 302–309 (2016). https://doi.org/10.1007/s00223-016-0144-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-016-0144-4