Abstract
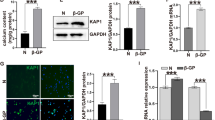
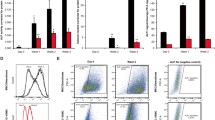
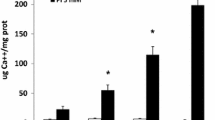
Vascular calcification is an important pathological condition associated with increased risk of cardiovascular mortality. Hydroxyapatite (HA) found in such deposits is the same polymorph of calcium (Ca) found in bone, indicating calcification may involve mechanisms akin to bone formation. Vascular smooth muscle cells (Vsmcs) have been shown to undergo phenotypic change to osteoblast-like cells. However, the mechanisms underlying this phenotypic change are unclear, and whether the stimulus to become osteogenic is a result of loss of mineralization inhibitors or early mineral deposits is not known. Our aim in this study is to identify mechanisms and signal transduction pathways that cause differentiation of Vsmcs into osteoblast-like cells in the presence of HA. We first characterized vascular origin of Vsmcs by studying the expression of smooth muscle cell markers: myosin heavy chain and smooth muscle actin along with SM22α at both mRNA and protein levels. Vsmcs grown on HA exhibited progressive change in cellular morphology at 3-, 7-, and 14-day time points. Culturing of Vsmcs on HA disc resulted in decrease in media Ca levels and increased expression of Ca-sensing receptor (CaSR) on Vsmcs resulting in upregulation of intracellular CaSR signaling leading to increased BMP-2 secretion. BMP-2 pathway mediated differentiation of Vsmcs to osteoblast-like cells shown by expression of osteogenic markers like runt-related transcription factor 2, osteocalcin, and alkaline phosphatase at mRNA and protein levels. Blocking CaSR by NPS-2143 reduced BMP-2 secretion and blocking the BMP-2 pathway by LDN-193189, a BMP inhibitor, modulated expression of osteogenic markers confirming their role in osteogenesis of Vsmcs.





Similar content being viewed by others
Abbreviations
- VSMCs:
-
Vascular smooth muscle cells
- GAPDH:
-
Glyceraldehyde 3-phosphate dehydrogenase
- SMA:
-
Alpha smooth muscle actin
- MHC:
-
Myosin heavy chain
- BMP-2:
-
Bone morphogenetic protein 2
- SMAD-5:
-
Sma- and Mad-related protein 5
- Col2a1:
-
Collagen type II alpha 1
- RUNX2:
-
Runt-related transcription factor 2
- ALP:
-
Alkaline phosphatase
- DAPI:
-
4′,6-Diamidino-2-phenylindole, dihydrochloride
- HA:
-
Hydroxyapatite
- MGP:
-
Matrix-gla protein
References
Roberto Wayhs, Allan Zelinger, Paolo Raggi (2002) High coronary artery calcium scores pose an extremely elevated risk for hard events. J Am Coll Cardiol 39(2):225–230
Yadon Arad, Louise Spadaro, Ken Goodman, David Newstein, Guerci AD (2000) Prediction of coronary events with electron beam computed tomography. J Am Coll Cardiol 36(4):1253–1260
London GM, Guerin AP, Marchais SJ, Métivier F, Pannier B, Adda H (2003) Arterial media calcification in end-stage renal disease: impact on all-cause and cardiovascular mortality. Nephrol Dial Transplant 18(9):1731–1740
Goodman WG, London G, Amann K, Block GA, Giachelli C, Hruska KA, Ketteler M, Levin A, Massy Z, McCarron DA, Raggi P, Shanahan CM, Yorioka N (2004) Vascular calcification in chronic kidney disease. Am J Kidney Dis 43(3):572–579
Johnson RC, Leopold JA, Loscalzo J (2006) Vascular calcification: pathobiological mechanisms and clinical implications. Circ Res 99(10):1044–1059
Price PA, Faus SA, Williamson MK (1998) Warfarin causes rapid calcification of the elastic lamellae in rat arteries and heart valves. Arterioscler Thromb Vasc Biol 18(9):1400–1407
Price P, William MK, Nguyen TMT, Than TN (2004) Serum levels of the fetuin-mineral complex correlate with artery calcification in the rat. J Biol Chem 279(3):1594–1600
Speer MY, McKee MD, Guldberg RE, Liaw L, Yang H-Y, Tung E, Karsenty G, Giachelli CM (2002) Inactivation of the Osteopontin gene enhances vascular calcification of matrix Gla protein–deficient mice: evidence for osteopontin as an inducible inhibitor of vascular calcification in vivo. J Exp Med 196(8):1047–1055
Watson KE, Bostrom K, Ravindranath R, Lam T, Norton B, Demer LL (1994) TGF-beta 1 and 25-hydroxycholesterol stimulate osteoblast-like vascular cells to calcify. J Clin Invest. 93(5):2106–2113
Bostrom MK, Watson KE, Horn S, Wortham C, Herman IM, Demer LL (1993) Bone morphogenetic protein expression in human atherosclerotic lesions. J Clin Investig 91(4):1800–1809
Reynolds JL, Joannides AJ, Skepper JN, McNair R, Schurgers LJ, Proudfoot D, Jahnen-Dechent W, Weissberg PL, Shanahan CM (2004) Human vascular smooth muscle cells undergo vesicle-mediated calcification in response to changes in extracellular calcium and phosphate concentrations: a potential mechanism for accelerated vascular calcification in ESRD. J Am Soc Nephrol 15(11):2857–2867
Hsueh Yang, Gabrielle Curinga, Giachelli CM (2004) Elevated extracellular calcium levels induce smooth muscle cell matrix mineralization in vitro. Int Soc Nephrol 66:6
Hruska KA, Mathew S, Saab G (2005) Bone morphogenetic proteins in vascular calcification. Circ Res 97(2):105–114
Zhou H, Hammonds RG Jr, Findlay DM, Martin TJ, Ng KW (1993) Differential effects of transforming growth factor-beta 1 and bone morphogenetic protein 4 on gene expression and differentiated function of preosteoblasts. J Cell Physiol 155(1):112–119
Wang EA, Israel ID, Kelly S, Luxenberg DP (1993) Bone morphogenetic protein-2 causes commitment and differentiation in C3H10T1/2 and 3T3 cells. Growth Factors 9(1):57–71
Byon CH, Javed A, Dai Q, Kappes JC, Clemens TL, Darley-Usmar VM, McDonald JM, Chen Y (2008) Oxidative stress induces vascular calcification through modulation of the osteogenic transcription factor Runx2 by AKT signaling. J Biol Chem 22:15319–15327
Ewence AE, Bootman M, Roderick HL, Skepper JN, McCarthy G, Epple M, Neumann M, Shanahan CM, Proudfoot D (2008) Calcium phosphate crystals induce cell death in human vascular smooth muscle cells: a potential mechanism in atherosclerotic plaque destabilization. Circ Res 5:28–34
Collett GDM, Sage AP, Kirton JP, Alexander MY, Gilmore AP, Canfield AE (2007) Axl/phosphatidylinositol 3-kinase signaling inhibits mineral deposition by vascular smooth muscle cells. Circ Res 100(4):502–509
Yang Lei, Aditi Sinha, Nasim Nosoudi, Ankit Grover, Naren Vyavahare (2014) Hydroxyapatite and calcified elastin induce osteoblast-like differentiation in rat aortic smooth muscle cells. Exp Cell Res 323(1):198–208
Orlandi A, Ehrlich HP, Ropraz P, Spagnoli LG, Gabbiani G (1994) Rat aortic smooth muscle cells isolated from different layers and at different times after endothelial denudation show distinct biological features in vitro. Arterioscler Thromb Vasc Biol 14(6):982–989
Shroff Rukshana, Long David A, Shanahan Catherine (2013) Mechanistic insights into vascular calcification in CKD. J Am Soc Nephrol 24:179–189
Cuny GD, Yu PB, Laha JK, Xing X, Liu J-F, Lai CS, Deng DY, Sachidanandan C, Bloch KD, Peterson RT (2008) Structure–activity relationship study of bone morphogenetic protein (BMP) signaling inhibitors. Bioorg Med Chem Lett 18(15):4388–4392
Covic A, Kanbay M, Voroneanu L, Turgut F, Serban DN, Serban IL, Goldsmith DJ (2010) Vascular calcification in chronic kidney disease. Clin Sci 119(3):111–121
Wang J, Zhou HY, Salih E, Xu L, Wunderlich L, Gu X, Hofstaetter JG, Torres M, Glimcher MJ (2006) Site-specific in vivo calcification and osteogenesis stimulated by bone sialoprotein. Calcif Tissue Int 79(3):179–189
Zhou Y, Wang J-Y, Feng H, Wang C, Li L, Wu D, Lei H, Li H, Wu L-L (2014) Overexpression of C1q/tumor necrosis factor–related protein-3 promotes phosphate-induced vascular smooth muscle cell calcification both in vivo and in vitro. Arterioscler Thromb Vasc Biol 34(5):1002–1010
Fitzpatrick LA, Severson A, Edwards WD, Ingram RT (1994) Diffuse calcification in human coronary arteries. Association of osteopontin with atherosclerosis. J Clin Investig 94(4):1597–1604
Zebboudj AF, Imura M, Bostrom K (2002) Matrix GLA protein, a regulatory protein for bone morphogenetic protein-2. J Biol Chem 277(6):4388–4394
Diane Proudfoot, Shanahan CM (2006) Molecular mechanisms mediating vascular calcification: role of matrix Gla protein (Review Article). Nephrology 11(5):455–461
Luo G, Ducy P, McKee MD, Pinero GJ, Loyer E, Behringer RR, Karsenty G (1997) Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature 386(6620):78–81
Chen NX, Duan D, O’Neill KD, Wolisi GO, Koczman JJ, LaClair R, Moe SM (2006) The mechanisms of uremic serum-induced expression of bone matrix proteins in bovine vascular smooth muscle cells. Kidney Int 70(6):1046–1053
Lee K-S, Kim H-J, Li Q-L, Chi X-Z, Ueta C, Komori T, Wozney JM, Kim E-G, Choi Je-Young RH-M, Bae S-C (2000) Runx2 is a common target of transforming growth factor β1 and bone morphogenetic protein 2, and cooperation between Runx2 and Smad5 induces osteoblast-specific gene expression in the pluripotent mesenchymal precursor cell line C2C12. Mol Cell Biol 20(23):8783–8792
Lau W, Ling P, Ashwini M, Sharon MG, Cecilia M (2011) Direct effects of phosphate on vascular cell function. Adv Chron Kidney Dis 18(2):105–112
Silver IA, Murrills RJ, Etherington DJ (1988) Microelectrode studies on the acid microenvironment beneath adherent macrophages and osteoclasts. Exp Cell Res 175(2):266–276
Chattopadhyay N, Yano S, Tfelt-Hansen J, Rooney P, Kanuparthi D, Bandyopadhyay S, Ren X, Terwilliger E, Brown EM (2004) Mitogenic action of calcium-sensing receptor on rat calvarial osteoblasts. Endocrinology 145(7):3451–3462
Dinithi Peiris, Ivan Pacheco, Craig Spencer, MacLeod RJ (2007) The extracellular calcium-sensing receptor reciprocally regulates the secretion of BMP-2 and the BMP antagonist Noggin in colonic myofibroblasts. Am J Physiol Gastrointest Liver Physiol 292:753–766
Barradas AMC, Fernandes HAM, Groen N, Chai YC, Schrooten J, van de Peppel J, van Leeuwen JPTM, van Blitterswijk CA, de Boer J (2012) A calcium-induced signaling cascade leading to osteogenic differentiation of human bone marrow-derived mesenchymal stromal cells. Biomaterials 33(11):3205–3215
Costa Jr JR, Abizaid A, Costa R, Feres F, Tanajura LF, Abizaid A, Mattos LA, Staico R, Siqueira D, Sousa A, Bonan GMR, Raoul Sousa JE (2008) Preliminary results of the hydroxyapatite nonpolymer-based sirolimus-eluting stent for the treatment of single de novo coronary lesions: a first-in-human analysis of a third-generation drug-eluting stent system. JACC 1(5):545–551
Acknowledgments
The authors would like to thank Jayesh Betala and Dr. Martine LaBerge at the Department of Bioengineering at Clemson University for the gift of rat aortic smooth muscle cells. This study was partially supported by NIH P20GM103444 grant and the Hunter Endowment to NV.
Conflict of Interest
Pranjal Nahar-Gohad, Neeraj Gohad, Chen-Chih Tsai, Rajendra Bordia, and Naren Vyavahare declare that there is no conflict of interest.
Human and Animal Rights and Informed Consent
No Human Subjects were involved in this study. The Clemson University Institutional Animal Care and Use Committee (IACUC) assures that animal care and use are in compliance with all federal, state and local regulations as well as University policy and assurances. This research study has been carried out in compliance with IACUC regulations.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nahar-Gohad, P., Gohad, N., Tsai, CC. et al. Rat Aortic Smooth Muscle Cells Cultured on Hydroxyapatite Differentiate into Osteoblast-Like Cells via BMP-2–SMAD-5 Pathway. Calcif Tissue Int 96, 359–369 (2015). https://doi.org/10.1007/s00223-015-9962-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-015-9962-z