Abstract
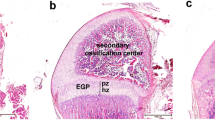
Endogenous estrogen has beneficial effects on mature bone and negatively affects the developing skeleton, whereas the effect of environmental estrogens is not known. Methoxychlor (MXC) is a synthetic estrogen known as a persistent organochlorine and used as a pesticide. Methoxychlor and its metabolites display estrogenic, anti-estrogenic and anti-androgenic activity and may therefore influence bone. Fifty-eight male fetal and neonatal rats were exposed to either: a negative control (DMSO), 0.020, 100 mg/kg MXC, or 1 mg/kg β-estradiol-3-benzoate (EB; positive control). Rats were treated daily for 11 days, from embryonic day 19 to postnatal day (PND) 7 or for 4 days during the postnatal period (PND 0–7). All rats were analyzed at PND-84. Total body, femur, spine, and tibia areal bone mineral density (BMD) and content (BMC), lean body mass (LBM) and fat were measured by dual energy X-ray absorptiometry. Bone geometry and volumetric (v) BMD were measured using micro-computed tomography and biomechanical properties using three-point bending were assessed. Rats exposed to EB or MXC (at either the high and/or low dose), independent of exposure interval showed lower body weight, LBM, tibia and femur BMD and length, and total body BMD and BMC than DMSO control group (p ≤ 0.05). Methoxychlor and EB exposure increased cortical porosity compared to DMSO controls. Trabecular vBMD, number and separation, and cortical polar moment of inertia and cross-sectional area were lower due to EB exposure compared to control (p < 0.05). Early MXC exposure compromises cortical porosity and bone size at maturity, and could ultimately increase the risk of fracture with aging.


Similar content being viewed by others
References
Crain DA, Janssen SJ, Edwards TM, Heindel J, Ho SM, Hunt P, Iguchi T, Juul A, McLachlan JA, Schwartz J, Skakkebaek N, Soto AM, Swan S, Walker C, Woodruff TK, Woodruff TJ, Giudice LC, Guillette LJ Jr (2008) Female reproductive disorders: the roles of endocrine-disrupting compounds and developmental timing. Fertil Steril 90:911–940
Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, Zoeller RT, Gore AC (2009) Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev 30:293–342
Bergman A, Heindel JJ, Kasten T, Kidd KA, Jobling S, Neira M, Zoeller RT, Becher G, Bjerregaard P, Bornman R, Brandt I, Kortenkamp A, Muir D, Drisse MN, Ochieng R, Skakkebaek NE, Bylehn AS, Iguchi T, Toppari J, Woodruff TJ (2013) The impact of endocrine disruption: a consensus statement on the state of the science. Environ Health Perspect 121:a104–a106
Agency for Toxic Substances and Disease Registry (ATSDR) (2002) Toxicological profile for methoxychlor. U.S. Department of Health and Human Services, Public Health, Atlanta
Giado KW, Manes SC, McDonnel DP, Dehal SS, Kupfer D, Safe S (2000) Interaction of methoxychlor and related compounds with estrogen receptor alpha and beta, and androgen receptor: structure–activity studies. Mol Pharmocol 58:852–858
Zama AM, Uzumcu M (2009) Fetal and neonatal exposure to an endocrine disruptor methoxychlor causes epigenetic alterations in adult avarian genes. Endocrinology 150:4681–4691
Armenti AE (2007) Methoxychlor exposure during fetal and neonatal periods of development affects adult ovarian function and female fertility in rats. Thesis, Rutgers University
Armenti AE, Zama AM, Passantino L, Uzumcu M (2008) Developmental methoxychlor exposure affects multiple reproductive parameters and ovarian: folliculogenesis and gene expression in adult rats. Toxicol Appl Pharmacol 233:286–296
Murono EP, Derk RC, Akgul Y (2005) In vivo exposure of young adult male rats to methoxychlor reduces serum testosterone levels and ex vivo Leydig cell testosterone formation and cholesterol side-chain cleavage activity. Reprod Toxicol 21:148–153
Zachow R, Uzumcu M (2006) The methoxychlor metabolite, 2,2-bis-(p-hydroxyphenyl)-1,1,1-trichloroethane, inhibits steroidogenesis in rat ovarian granulosa cells in vitro. Reprod Toxicol 22:659–665
Basavarajappa MS, Craig ZR, Hernandez-Ochoa I, Paulose T, Leslie TC, Flaws JA (2011) Methoxychlor reduces estradiol levels by altering steroidogenesis and metabolism in mouse antral follicles in vitro. Toxicol Appl Pharmacol 253:161–169
Manikkam M, Haque MM, Guerrero-Bosagna C, Nilsson EE, Skinner MK (2014) Pesticide methoxychlor promotes the epigenetic transgenerational inheritance of adult-onset disease through the female germline. PLoS One 9:e102091
Anway MD, Cupp AS, Uzumcu M, Skinner MK (2005) Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science 308:1466–1469
Hotchkiss CE, Weis C, Blaydes B, Newbold R, Delclos KB (2008) Multigenerational exposure to ethinyl estradiol affects bone geometry, but not bone mineral density in rats. Bone 43:110–118
Márquez Hernández RA, Ohtani J, Fujita T, Sunagawa H, Kawata T, Kaku M, Motokawa M, Tanne K (2011) Sex hormones receptors play a crucial role in the control of femoral and mandibular growth in newborn mice. Eur J Orthod 33:564–569
Boettger-Tong H, Murthy L, Chiappetta C, Kirkland JL, Goodwin B, Adlercreutz H, Stancel GM, Makela S (1998) A case of a laboratory animal feed with high estrogenic activity and its impact on vivo responses to exogenously administered estrogens. Environ Health Perspect 106:369–373
Xue N, Xu X, Jin Z (2005) Screening 31 endocrine-disrupting pesticides in water and surface sediment samples from Beijing Guanting reservoir. Chemosphere 61:1594–1606
Bempah CK, Donkor AK (2011) Pesticide residues in fruits at the market level in Accra Metropolis, Ghana, a preliminary study. Environ Monit Assess 175:551–561
Oh CH (2009) Monitoring of residual pesticides in herbal drug materials of Korea and China. Bull Environ Contam Toxicol 8:639–643
Gray LE Jr, Ostby J, Ferrell J, Rehnberg G, Linder R, Cooper R, Goldman J, Slott V, Laskey J (1989) A dose–response analysis of methoxychlor-induced alterations of reproductive development and function in the rat. Fundam Appl Toxicol 12:92–108
Chapin RE, Harris MW, Davis BJ, Ward SM, Wilson RE, Mauney MA, Lockhart AC, Smialowicz RJ, Moser VC, Burka LT, Collins BJ (1997) The effects of perinatal/juvenile methoxychlor exposure on adult rat nervous, immune, and reproductive system function. Fundam Appl Toxicol 40:138–157
Uzumcu M, Kuhn PE, Marano JE, Armenti AE, Passantino L (2006) Early postnatal methoxychlor exposure inhibits folliculogenesis and stimulates anti-Mullerian hormone production in the rat ovary. J Endocrinol 191:549–558
Deroo BJ, Korach S (2006) Estrogen receptors and human disease. J Clin Invest. 116:561–570
Golub MS, Hogrefe CE, Germann SL, Jerome CP (2004) Endocrine disruption in adolescence: immunologic, hematologic, and bone effects in monkeys. Toxicol Sci 82:598–607
Migliaccio S, Newbold RR, McLachlan JA, Korach KS (1995) Alterations in estrogen levels during development affects the skeleton: use of an animal model. Environ Health Perspect 7:95–97
Migliaccio S, Newbold RR, Bullock BC, Jefferson WJ, Sutton FG Jr, McLachlan JA, Korach KS (1996) Alterations of maternal estrogen levels during gestation affect the skeleton of female offspring. Endocrinology 137:2118–2125
Pelch KE, Carleton SM, Phillips CL, Nagel SC (2012) Developmental exposure to xenoestrogens at low doses alters femur length and tensile strength in adult mice. Biol Reprod 86:69
Kaludjerovic J, Ward WE (2008) Diethylstilbesterol has gender-specific effects on weight gain and bone development in mice. J Toxicol Environ Health A 71:1032–1042
Rowas SA, Haddad R, Gawri R, Al Ma’awi AA, Chalifour LE, Antoniou J, Mwale F (2012) Effect of in utero exposure to diethylstilbestrol on lumbar and femoral bone, articular cartilage, and the intervertebral disc in male and female adult mice progeny with and without swimming exercise. Arthritis Res Ther 14:R17
Gaido KW, Leonard LS, Maness SC, Hall JM, McDonnell DP, Saville B, Safe S (1999) Differential interaction of the methoxychlor metabolite 2,2-bis-(p-hydroxyphenyl)-1,1,1-trichloroethane with estrogen receptors alpha and beta. Endocrinology 140:5746–5753
Gore AC, Walker DM, Zama AM, Armenti AE, Uzumcu M (2011) Early life exposure to endocrine-disrupting chemicals causes lifelong molecular reprogramming of the hypothalamus and premature reproductive aging. Mol Endocrinol 25:2157–2168
Dodge JA, Glasebrook AL, Magee DE, Phillips DL, Sato M, Short LL, Bryant HU (1996) Environmental estrogens: effects on cholesterol lowering and bone in the ovariectomized rat. J Steroid Biochem Mol Biol 59:155–161
Acknowledgments
We wish to thank Dr. LC Pop for reviewing this manuscript, Dr. Schlussel for her statistical advice, AE Armenti for assisting with the in vivo experimental procedures in this study, and E Bandali for her assistance with the bone images. This study is supported by NJAES (0153866) to SAS and NIH Grant (ES013854) to MU.
Conflict of interest
Heather S. Fagnant, Mehmet Uzumcu, Patricia Buckendahl, Michael G. Dunn, Peter Shupper and Sue A. Shapses declare no conflict of interest.
Human and Animal Rights and Informed Consent
The procedures in this study were approved and conducted in accordance with the guidelines of the Rutgers University Institutional Animal Care and Use Committee.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fagnant, H.S., Uzumcu, M., Buckendahl, P. et al. Fetal and Neonatal Exposure to the Endocrine Disruptor, Methoxychlor, Reduces Lean Body Mass and Bone Mineral Density and Increases Cortical Porosity. Calcif Tissue Int 95, 521–529 (2014). https://doi.org/10.1007/s00223-014-9916-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-014-9916-x