Abstract
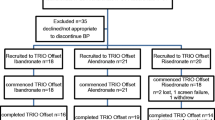
Compliance to osteoporosis treatment with oral bisphosphonates is very poor. Intermittent intravenous bisphosphonate is a useful alternative, but this route is not readily available. Neridronate, a nitrogen-containing bisphosphonate that can be given intramuscularly (IM), was tested in a phase 2 clinical trial in 188 postmenopausal osteoporotic women randomized to IM treatment with 25 mg neridronate every 2 weeks, neridronate 12.5 or 25 mg every 4 weeks, or placebo. All patients received calcium and vitamin D supplements. The patients were treated over 12 months with 2-year posttreatment follow-up. After 12-month treatment, all three doses were associated with significant bone mineral density (BMD) increases at both the total hip and spine. A significant dose–response relationship over the three doses was observed for the BMD changes at the total hip but not at the spine. Bone alkaline phosphatase decreased significantly by 40–55% in neridronate-treated patients, with an insignificant dose–response relationship. Serum type I collagen C-telopeptide decreased by 58–79%, with a significant dose–response relationship (P < 0.05). Two years after treatment discontinuation, BMD declined by 1–2% in each dose group, with values still significantly higher than baseline at both the spine and the total hip. Bone turnover markers progressively increased after treatment discontinuation, and on the second year of follow-up the values were significantly higher than pretreatment baseline. The results of this study indicate that IM neridronate might be of value for patients intolerant to oral bisphosphonates and unwilling or unable to undergo intravenous infusion of bisphosphonates.


Similar content being viewed by others
References
Black DM, Thompson DE, Bauer DC, Ensrud K, Musliner T, Hochberg MC, Nevitt MC, Suryawanshi S, Cummings SR, Fracture Intervention Trial (2000) Fracture risk reduction with alendronate in women with osteoporosis: the Fracture Intervention Trial FIT Research Group. J Clin Endocrinol Metab 85:4118–4124
McClung MR, Geusens P, Miller PD, Zippel H, Bensen WG, Roux C, Adami S, Fogelman I, Diamond T, Eastell R, Meunier PJ, Reginster JY, Hip Intervention Program Study Group (2001) Effect of risedronate on the risk of hip fracture in elderly women. N Engl J Med 344:333–340
Chesnut CH III, Skag A, Christiansen C, Recker R, Stakkestad JA, Hoiseth A, Felsenberg D, Huss H, Gilbride J, Schimmer RC, Delmas PD (2004) Oral Ibandronate Osteoporosis Vertebral Fracture Trial in North America and Europe (BONE). Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res 19:1241–1249
Delmas PD, Adami S, Strugala C, Stakkestad JA, Reginster JY, Felsenberg D, Christiansen C, Civitelli R, Drezner MK, Recker RR, Bolognese M, Hughes C, Masanauskaite D, Ward P, Sambrook P, Reid DM (2006) Intravenous ibandronate injections in postmenopausal women with osteoporosis: one-year results from the dosing intravenous administration study. Arthritis Rheum 54:1838–1846
Black DM, Delmas PD, Eastell R, Reid IR, Boonen S, Cauley JA, Cosman F, Lakatos P, Leung PC, Man Z, Mautalen C, Mesenbrink P, Hu H, Caminis J, Tong K, Rosario-Jansen T, Krasnow J, Hue TF, Sellmeyer D, Eriksen EF, Cummings SR, HORIZON Pivotal Fracture Trial (2007) Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 356:1809–1822
Rossini M, Braga V, Gatti D, Gerardi D, Zamberlan N, Adami S (1999) Intramuscular clodronate therapy in postmenopausal osteoporosis. Bone 24:125–129
Adami S, Bevilacqua M, Broggini M, Filipponi P, Ortolani S, Palummeri E, Ulivieri F, Nannipieri F, Braga V (2002) Short-term intravenous therapy with neridronate in Paget’s disease. Clin Exp Rheumatol 20:55–58
Adami S, Gatti D, Colapietro F, Fracassi E, Braga V, Rossini M, Tatò L (2003) Intravenous neridronate in adults with osteogenesis imperfecta. J Bone Miner Res 18:126–130
Braga V, Gatti D, Colapietro F, Battaglia E, Righetti D, Prizzi R, Rossini M, Adami S (2003) Intravenous intermittent neridronate in the treatment of postmenopausal osteoporosis. Bone 33:342–345
Hui SL, Gao SJ, Zhou XH, Zhou XH, Johnston CC, Lu Y, Gluer CC, Grampp S, Genant H (1997) Universal standardization of bone density measurements: a method with optimal properties for calibration among several instruments. J Bone Miner Res 12:1463–1470
Adami S, Bianchi G, Brandi ML, Giannini S, Ortolani S, DiMunno O, Frediani B, Rossini M, on behalf of the BONTURNO study group (2008) Determinants of bone turnover markers in healthy premenopausal women. Calcif Tissue Int 82:341–347
Adami S, Bhalla AK, Dorizzi R, Montesanti F, Rosini S, Salvagno G, Lo Cascio V (1987) The acute-phase response after bisphosphonate administration. Calcif Tissue Int 41:326–331
Rosen CJ, Hochberg MC, Bonnick SL, McClung M, Miller P, Broy S, Kagan R, Chen E, Petruschke RA, Thompson DE, de Papp AE, Fosamax Actonel Comparison Trial Investigators (2005) Treatment with once-weekly alendronate 70 mg compared with once-weekly risedronate 35 mg in women with postmenopausal osteoporosis: a randomized double-blind study. J Bone Miner Res 20:141–151
Reginster JY, Adami S, Lakatos P, Greenwald M, Stepan JJ, Silverman SL, Christiansen C, Rowell L, Mairon N, Bonvoisin B, Drezner MK, Emkey R, Felsenberg D, Cooper C, Delmas PD, Miller PD (2006) Efficacy and tolerability of once-monthly oral ibandronate in postmenopausal osteoporosis: 2 year results from the MOBILE study. Ann Rheum Dis 65:654–661
Saag K, Lindsay R, Kriegman A, Beamer E, Zhou W (2007) A single zoledronic acid infusion reduces bone resorption markers more rapidly than weekly oral alendronate in postmenopausal women with low bone mineral density. Bone 40:1238–1243
Black DM, Schwartz AV, Ensrud KE, Cauley JA, Levis S, Quandt SA, Satterfield S, Wallace RB, Bauer DC, Palermo L, Wehren LE, Lombardi A, Santora AC, Cummings SR, FLEX Research Group (2006) Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA 296:2927–2938
McClung MR, Wasnich RD, Hosking DJ, Christiansen C, Ravn P, Wu M, Mantz AM, Yates J, Ross PD, Santora AC 2nd, Early Postmenopausal Intervention Cohort Study (2004) Prevention of postmenopausal bone loss: six-year results from the Early Postmenopausal Intervention Cohort Study. J Clin Endocrinol Metab 89:4879–4885
Michalská D, Stepan JJ, Basson BR, Pavo I (2006) The effect of raloxifene after discontinuation of long-term alendronate treatment of postmenopausal osteoporosis. J Clin Endocrinol Metab 91:870–877
Nancollas GH, Tang R, Phipps RJ, Henneman Z, Gulde S, Wu W, Mangood A, Russell RG, Ebetino FH (2006) Novel insights into actions of bisphosphonates on bone: differences in interactions with hydroxyapatite. Bone 38:617–627
Adami S, Kanis JA (1995) Assessment of involutional bone loss: methodological and conceptual problems. J Bone Miner Res 10:511–517
Roschger P, Rinnerthaler S, Yates J, Rodan GA, Fratzl P, Klaushofer K (2001) Alendronate increases degree and uniformity of mineralization in cancellous bone and decreases the porosity in cortical bone of osteoporotic women. Bone 29:185–191
Brookhart MA, Avorn J, Katz JN, Finkelstein JS, Arnold M, Polinski JM, Patrick AR, Mogun H, Solmon DH (2007) Gaps in treatment among users of osteoporosis medications: the dynamics of non-compliance. Am J Med 120:251–256
Acknowledgment
The study was designed by one of the authors (S. A.) and fully supported by an unlimited grant from Abiogen Pharma (Pisa, Italy).
Author information
Authors and Affiliations
Corresponding author
Appendix: Centers That Participated in the Study
Appendix: Centers That Participated in the Study
Riabilitazione Reumatologica, Università di Verona (S. Adami, M. Rossini); U.O. Medicina D, Università di Verona (V. Lo Cascio, F. Bertoldo); Clinica Medica I, Università di Padova (G. Realdi, L. Sartori); U.O. Reumatologia, Università Di Pisa (O. Di Munno, A. Delle Sedie); Ospedale di Umbertide (P. Filippini, S. Cristallini); Dipartimento Endocrinologia e Metabolismo, Università di Pisa (C. Marcocci, E. Vignali); Policlinico Le Scotte, Università di Siena (R. Marcolongo, B. Frediani); Ospedale Galliera, Genova (E. Palummeri, A. Barone); Clinica Medica I, Università di Catania (C.E. Fiore, P. Pennisi); Clinica Medica, Università di Parma (D. Costi).
Rights and permissions
About this article
Cite this article
Adami, S., Gatti, D., Bertoldo, F. et al. Intramuscular Neridronate in Postmenopausal Women with Low Bone Mineral Density. Calcif Tissue Int 83, 301–307 (2008). https://doi.org/10.1007/s00223-008-9179-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-008-9179-5