Abstract
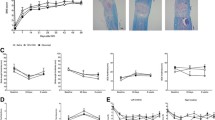
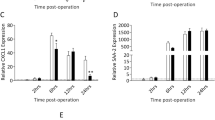
Systemic administration of a Connexin43 mimetic peptide, Peptide5, has been shown to reduce secondary tissue damage and improve functional recovery after spinal cord injury (SCI). This study investigated safety measures and potential off-target effects of Peptide5 systemic administration. Rats were subjected to a mild contusion SCI using the New York University impactor. One cohort was injected intraperitoneally with a single dose of fluorescently labelled Peptide5 and euthanised at 2 or 4 h post-injury for peptide distribution analysis. A second cohort received intraperitoneal injections of Peptide5 or a scrambled peptide and was culled at 8 or 24 h post-injury for the analysis of connexin proteins and systemic cytokine profile. We found that Peptide5 did not cross the blood-spinal cord barrier in control animals, but reached the lesion area in the spinal cord-injured animals without entering non-injured tissue. There was no evidence that the systemic administration of Peptide5 modulates Connexin43 protein expression or hemichannel closure in the heart and lung tissue of SCI animals. The expression levels of other major connexin proteins including Connexin30 in astrocytes, Connexin36 in neurons and Connexin47 in oligodendrocytes were also unaltered by systemic delivery of Peptide5 in either the injured or non-injured spinal cords. In addition, systemic delivery of Peptide5 had no significant effect on the plasma levels of cytokines, chemokines or growth factors. These data indicate that the systemic delivery of Peptide5 is unlikely to cause any off-target or adverse effects and may thus be a safe treatment option for traumatic SCI.







Similar content being viewed by others
References
Abdelaziz RR, Elkashef WF, Said E (2016) Tadalafil reduces airway hyperactivity and protects against lung and respiratory airways dysfunction in a rat model of silicosis. Int Immunopharmacol 40:530–541
Abudara V et al (2014) The connexin43 mimetic peptide Gap19 inhibits hemichannels without altering gap junctional communication in astrocytes. Front cell Neurosci 8:306
Aubert N, Ameller T, Legrand J-J (2012) Systemic exposure to parabens: pharmacokinetics, tissue distribution, excretion balance and plasma metabolites of [14C]-methyl-, propyl-and butylparaben in rats after oral, topical or subcutaneous administration. Food Chem Toxicol 50:445–454
Baek M et al (2012) Pharmacokinetics, tissue distribution, and excretion of zinc oxide nanoparticles. Int J Nanomed 7:3081–3097
Bilgen M, Dogan B, Narayana PA (2002) In vivo assessment of blood-spinal cord barrier permeability: serial dynamic contrast enhanced MRI of spinal cord injury. Magn Reson Imaging 20:337–341
Cesta MF (2006) Normal structure, function, and histology of the spleen. Toxicol Pathol 34:455–465
Chang H-M, Cheng J-C, Leung PC (2013) Theca-derived BMP4 and BMP7 down-regulate connexin43 expression and decrease gap junction intercellular communication activity in immortalized human granulosa cells. J Clin Endocrinol Metab 98:E437–E445
Chen YS, Green CR, Wang K, Danesh-Meyer HV, Rupenthal ID (2014) Sustained intravitreal delivery of connexin43 mimetic peptide by poly (d, l-lactide-co-glycolide) acid micro-and nanoparticles–closing the gap in retinal ischaemia. Eur J Pharm Biopharm. doi:10.1016/j.ejpb.2014.12.005
Chen YS, Green CR, Teague R, Perrett J, Danesh-Meyer HV, Toth I, Rupenthal ID (2015) Intravitreal injection of lipoamino acid-modified connexin43 mimetic peptide enhances neuroprotection after retinal ischemia. Drug Deliv Transl Res 5:480–488
Chevallier D, Carette D, Segretain D, Gilleron J, Pointis G (2013) Connexin 43 a check-point component of cell proliferation implicated in a wide range of human testis diseases. Cell Mol Life Sci 70:1207–1220
Cho W-S, Kang B-C, Lee JK, Jeong J, Che J-H, Seok SH (2013) Comparative absorption, distribution, and excretion of titanium dioxide and zinc oxide nanoparticles after repeated oral administration. Part Fibre Toxicol 10:1
Danesh-Meyer HV et al (2012) Connexin43 mimetic peptide reduces vascular leak and retinal ganglion cell death following retinal ischaemia. Brain 135:506–520
Davidson JO et al (2012a) Connexin hemichannel blockade improves outcomes in a model of fetal ischemia. Ann Neurol 71:121–132
Davidson JO, Green CR, Nicholson LF, Bennet L, Gunn AJ (2012b) Deleterious effects of high dose connexin 43 mimetic peptide infusion after cerebral ischaemia in near-term fetal sheep. Int J Mol Sci 13:6303–6319
Davidson J, Green C, Nicholson L, Bennet L, Gunn A (2013a) Connexin hemichannel blockade is neuroprotective after, but not during, global cerebral ischemia in near-term fetal sheep. Exp Neurol 248:301–308
Davidson J, Green CR, Bennet L, Nicholson LFB, Danesh-Meyer H, O’Carroll SJ, Gunn AJ (2013b) A key role for connexin hemichannels in spreading ischemic brain injury. Curr Drug Targets 14:36–46
Davidson JO, Drury PP, Green CR, Nicholson LF, Bennet L, Gunn AJ (2014) Connexin hemichannel blockade is neuroprotective after asphyxia in preterm fetal sheep. PLoS ONE 9:e96558
Davidson J, Green C, Bennet L, Gunn A (2015) Battle of the hemichannels–connexins and pannexins in ischemic brain injury. Int J Dev Neurosci 45:66–74
Decrock E et al (2015) Connexin and pannexin signaling pathways, an architectural blueprint for CNS physiology and pathology? Cell Mol Life Sci 72:2823–2851
Deng Z-H et al (2014) Inhibition of the connexin 43 elevation may be involved in the neuroprotective activity of leptin against brain ischemic injury. Cell Mol Neurobiol 34:871–879
Dorshkind K, Green L, Godwin A, Fletcher W (1993) Connexin-43-type gap junctions mediate communication between bone marrow stromal cells. Blood 82:38–45
Dürig J et al (2000) Intercellular communication between bone marrow stromal cells and CD34+ haematopoietic progenitor cells is mediated by connexin 43-type gap junctions. Br J Haematol 111:416–425
Eloranta M, Alm G (1999) Splenic marginal metallophilic macrophages and marginal zone macrophages are the major interferon-/producers in mice upon intravenous challenge with herpes simplex virus. Scand J Immunol 49:391–394
Faigle M, Seessle J, Zug S, El Kasmi KC, Eltzschig HK (2008) ATP release from vascular endothelia occurs across Cx43 hemichannels and is attenuated during hypoxia. PLoS ONE 3:e2801
Figley SA, Khosravi R, Legasto JM, Tseng Y-F, Fehlings MG (2014) Characterization of vascular disruption and blood–spinal cord barrier permeability following traumatic spinal cord injury. J Neurotrauma 31:541–552
Gauter-Fleckenstein B et al (2014) Robust rat pulmonary radioprotection by a lipophilic Mn N-alkylpyridylporphyrin, MnTnHex-2-PyP 5+. Redox Biol 2:400–410
Gordh T, Chu H, Sharma HS (2006) Spinal nerve lesion alters blood–spinal cord barrier function and activates astrocytes in the rat. Pain 124:211–221
Gourdie RG, Severs NJ, Green CR, Rothery S, Germroth P, Thompson RP (1993) The spatial distribution and relative abundance of gap-junctional connexin40 and connexin43 correlate to functional properties of components of the cardiac atrioventricular conduction system. J Cell Sci 105:985–991
Gu Y, Wu G, Wang X, Wang X, Wang Y, Huang C (2014) Artemisinin prevents electric remodeling following myocardial infarction possibly by upregulating the expression of connexin 43. Mol Med Rep 10:1851–1856
Guo H, Acevedo P, Parsa FD, Bertram JS (1992) Gap-junctional protein connexin 43 is expressed in dermis and epidermis of human skin: differential modulation by retinoids. J Investig Dermatol 99:460–467
Habgood M et al (2007) Changes in blood–brain barrier permeability to large and small molecules following traumatic brain injury in mice. Eur J Neurosci 25:231–238
Han S et al (2010) Rescuing vasculature with intravenous angiopoietin-1 and αvβ3 integrin peptide is protective after spinal cord injury. Brain 133:1026–1042
Hawat G, Benderdour M, Rousseau G, Baroudi G (2010) Connexin 43 mimetic peptide Gap26 confers protection to intact heart against myocardial ischemia injury. Pflüg Archiv-Eur J Physiol 460:583–592
Hawat G, Hélie P, Baroudi G (2012) Single intravenous low-dose injections of connexin 43 mimetic peptides protect ischemic heart in vivo against myocardial infarction. J Mol Cell Cardiol 53:559–566
Ishikawa S et al (2012) Role of connexin-43 in protective PI3 K-Akt-GSK-3β signaling in cardiomyocytes. Am J Physiol Heart Circ Physiol 302:H2536–H2544
Jasim DA, Ménard-Moyon C, Bégin D, Bianco A, Kostarelos K (2015) Tissue distribution and urinary excretion of intravenously administered chemically functionalized graphene oxide sheets. Chem Sci 6:3952–3964
Kamasawa N, Sik A, Morita M, Yasumura T, Davidson K, Nagy J, Rash J (2005) Connexin-47 and connexin-32 in gap junctions of oligodendrocyte somata, myelin sheaths, paranodal loops and Schmidt-Lanterman incisures: implications for ionic homeostasis and potassium siphoning. Neuroscience 136:65–86
Kim Y et al (2017) Characterizing the mode of action of extracellular Connexin43 channel blocking mimetic peptides in an in vitro ischemia injury model. Biochim Biophys Acta Gen Subj 1861:68–78
Kleopa KA, Orthmann JL, Enriquez A, Paul DL, Scherer SS (2004) Unique distributions of the gap junction proteins connexin29, connexin32, and connexin47 in oligodendrocytes. Glia 47:346–357
Koulakoff A, Ezan P, Giaume C (2008) Neurons control the expression of connexin 30 and connexin 43 in mouse cortical astrocytes. Glia 56:1299–1311
Lampe PD, TenBroek EM, Burt JM, Kurata WE, Johnson RG, Lau AF (2000) Phosphorylation of connexin43 on serine368 by protein kinase C regulates gap junctional communication. J Cell Biol 149:1503–1512
Lee IH, Lindqvist E, Kiehn O, Widenfalk J, Olson L (2005) Glial and neuronal connexin expression patterns in the rat spinal cord during development and following injury. J Comp Neurol 489:1–10. doi:10.1002/cne.20567
Leybaert L, Braet K, Vandamme W, Cabooter L, Martin PE, Evans WH (2003) Connexin channels, connexin mimetic peptides and ATP release. Cell Commun Adhes 10:251–257
Li X et al (2004) Connexin47, connexin29 and connexin32 co-expression in oligodendrocytes and Cx47 association with zonula occludens-1 (ZO-1) in mouse brain. Neuroscience 126:611–630
Li MW, Mruk DD, Lee WM, Cheng CY (2010) Connexin 43 is critical to maintain the homeostasis of the blood–testis barrier via its effects on tight junction reassembly. Proc Natl Acad Sci 107:17998–18003
Liang L, Liu X, Wang Q, Cheng S, Zhang S, Zhang M (2013) Pharmacokinetics, tissue distribution and excretion study of resveratrol and its prodrug 3, 5, 4′-tri-O-acetylresveratrol in rats. Phytomedicine 20:558–563
Lin JH et al (1998) Gap-junction-mediated propagation and amplification of cell injury. Nat Neurosci 1:494–500
Lopes-Carvalho T, Kearney JF (2004) Development and selection of marginal zone B cells. Immunol Rev 197:192–205
Lund U, Rippe A, Venturoli D, Tenstad O, Grubb A, Rippe B (2003) Glomerular filtration rate dependence of sieving of albumin and some neutral proteins in rat kidneys. Am J Physiol Renal Physiol 284:F1226–F1234
Mao Y et al (2017) Systemic administration of Connexin43 mimetic peptide improves functional recovery after traumatic spinal cord Injury in adult Rats. J Neurotrauma 33:707–719. doi:10.1089/neu.2016.4625
Márquez-Rosado L, Solan JL, Dunn CA, Norris RP, Lampe PD (2012) Connexin43 phosphorylation in brain, cardiac, endothelial and epithelial tissues. Biochim Biophys Acta (BBA) Biomembr 1818:1985–1992
Mebius RE, Kraal G (2005) Structure and function of the spleen. Nat Rev Immunol 5:606–616
Nagy JI, Patel D, Ochalski PA, Stelmack GL (1999) Connexin30 in rodent, cat and human brain: selective expression in gray matter astrocytes, co-localization with connexin43 at gap junctions and late developmental appearance. Neuroscience 88:447–468
Nakase T, Söhl G, Theis M, Willecke K, Naus CC (2004) Increased apoptosis and inflammation after focal brain ischemia in mice lacking connexin43 in astrocytes. Am J Pathol 164:2067–2075
O’Carroll SJ, Gorrie CA, Velamoor S, Green CR, Nicholson LF (2013) Connexin43 mimetic peptide is neuroprotective and improves function following spinal cord injury. Neurosci Res 75:256–267
O’Carroll SJ, Alkadhi M, Nicholson LF, Green CR (2008) Connexin43 mimetic peptides reduce swelling, astrogliosis, and neuronal cell death after spinal cord injury. Cell Commun Adhes 15:27–42
Orellana JA et al (2011) ATP and glutamate released via astroglial connexin 43 hemichannels mediate neuronal death through activation of pannexin 1 hemichannels. J Neurochem 118:826–840
Orthmann-Murphy JL, Abrams CK, Scherer SS (2008) Gap junctions couple astrocytes and oligodendrocytes. J Mol Neurosci 35:101–116
Popovich PG, Horner PJ, Mullin BB, Stokes BT (1996) A quantitative spatial analysis of the blood–spinal cord barrier: I. Permeability changes after experimental spinal contusion injury. Exp Neurol 142:258–275
Rash JE, Yasumura T, Dudek F (1998) Ultrastructure, histological distribution, and freeze-fracture immunocytochemistry of gap junctions in rat brain and spinal cord. Cell Biol Int 22:731–749
Rash J, Staines W, Yasumura T, Patel D, Furman C, Stelmack G, Nagy J (2000) Immunogold evidence that neuronal gap junctions in adult rat brain and spinal cord contain connexin-36 but not connexin-32 or connexin-43. Proc Natl Acad Sci 97:7573–7578
Rash J, Yasumura T, Davidson K, Furman C, Dudek F, Nagy J (2001) Identification of cells expressing Cx43, Cx30, Cx26, Cx32 and Cx36 in gap junctions of rat brain and spinal cord. Cell Commun Adhes 8:315–320
Rottlaender D et al (2010) Connexin 43 acts as a cytoprotective mediator of signal transduction by stimulating mitochondrial K ATP channels in mouse cardiomyocytes. J Clin Investig 120:1441–1453
Rummery NM, Hickey H, McGurk G, Hill CE (2002) Connexin37 is the major connexin expressed in the media of caudal artery. Arterioscler Thromb Vasc Biol 22:1427–1432
Sandoval RM et al (2012) Multiple factors influence glomerular albumin permeability in rats. J Am Soc Nephrol 23:447–457
Scemes E, Suadicani SO, Spray DC (2000) Intercellular communication in spinal cord astrocytes: fine tuning between gap junctions and P2 nucleotide receptors in calcium wave propagation. J Neurosci 20:1435–1445
Soubeyrand M, Badner A, Vawda R, Chung YS, Fehlings MG (2014) Very high resolution ultrasound imaging for real-time quantitative visualization of vascular disruption after spinal cord injury. J Neurotrauma 31:1767–1775
Steger K, Tetens F, Bergmann M (1999) Expression of connexin 43 in human testis. Histochem Cell Biol 112:215–220
Stehberg J et al (2012) Release of gliotransmitters through astroglial connexin 43 hemichannels is necessary for fear memory consolidation in the basolateral amygdala. FASEB J 26:3649–3657
Tada J, Hashimoto K (1997) Ultrastructural localization of gap junction protein connexin 43 in normal human skin, basal cell carcinoma, and squamous cell carcinoma. J Cutan Pathol 24:628–635
Theriault E, Frankenstein U, Hertzberg E, Nagy J (1997) Connexin43 and astrocytic gap junctions in the rat spinal cord after acute compression injury. J Compar Neurol 382:199–214
Thompson RJ, Zhou N, MacVicar BA (2006) Ischemia opens neuronal gap junction hemichannels. Science 312:924–927
Tonkin RS, Mao Y, O’Carroll SJ, Nicholson LF, Green CR, Gorrie CA, Moalem-Taylor G (2015) Gap junction proteins and their role in spinal cord injury. Front Mol Neurosci 7:102. doi:10.3389/fnmol.2014.00102
van den Brule S et al (2014) Nanometer-long Ge-imogolite nanotubes cause sustained lung inflammation and fibrosis in rats. Part Fibre Toxicol 11:67
Van Kempen M, Fromaget C, Gros D, Moorman A, Lamers W (1991) Spatial distribution of connexin43, the major cardiac gap junction protein, in the developing and adult rat heart. Circ Res 68:1638–1651
van Rooijen N (1990) Antigen processing and presentation in vivo: the microenvironment as a crucial factor. Immunol Today 11:436–439
Venkatachalam MA, Rennke HG (1978) The structural and molecular basis of glomerular filtration. Circ Res 43:337–347
Yamamoto T, Ochalski A, Hertzberg E, Nagy J (1990) LM and EM immunolocalization of the gap junctional protein connexin 43 in rat brain. Brain Res 508:313–319
Yates C, Charlesworth A, Allen S, Reese N, Skinner R, Garcia-Rill E (2008) The onset of hyperreflexia in the rat following complete spinal cord transection. Spinal Cord 46:798–803
Yates C, Garrison K, Reese N, Charlesworth A, Garcia-Rill E (2011) Novel mechanism for hyper-reflexia and spasticity. Prog Brain Res 188:167
Chang Y-C, Chang H-Y, Hahn R, Lee E, Svoboda K, Gordon M, Gerecke D (2015) Connexin 43 antisense therapy modulates connexin expression and wound repair in nitrogen mustard injured mouse skin. FASEB J 29:873–876
Ye ZC, Wyeth MS, Baltan-Tekkok S, Ransom BR (2003) Functional hemichannels in astrocytes: a novel mechanism of glutamate release. J Neurosci 23:3588–3596
Yoon JJ, Green CR, O’Carroll SJ, Nicholson LF (2010) Dose-dependent protective effect of connexin43 mimetic peptide against neurodegeneration in an ex vivo model of epileptiform lesion. Epilepsy Res 92:153–162
Zhang C, Li Y, Chen J, Gao Q, Zacharek A, Kapke A, Chopp M (2006) Bone marrow stromal cells upregulate expression of bone morphogenetic proteins 2 and 4, gap junction protein connexin-43 and synaptophysin after stroke in rats. Neuroscience 141:687–695
Acknowledgements
This work was supported by the Australasian Spinal Cord Injury Network and the CatWalk Spinal Cord Injury Trust New Zealand. YM was supported by a University of Technology Sydney Doctoral Scholarship and an Australian Government Research Training Program Scholarship.
Author information
Authors and Affiliations
Contributions
Prof. Colin Green is a founding scientist of CoDa Therapeutics, Inc. (USA) and OcuNexus, Inc. (USA) which hold intellectual property rights related to Peptide5. For the remaining authors, no competing financial interests exist.
Corresponding author
Rights and permissions
About this article
Cite this article
Mao, Y., Nguyen, T., Tonkin, R.S. et al. Characterisation of Peptide5 systemic administration for treating traumatic spinal cord injured rats. Exp Brain Res 235, 3033–3048 (2017). https://doi.org/10.1007/s00221-017-5023-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-017-5023-3