Abstract
Previous studies examining discrete movements of Parkinson’s disease (PD) patients have found that in addition to performing movements that were slower than those of control participants, they exhibit specific deficits in movement coordination and in sensorimotor integration required to accurately guide movements. With medication, movement speed was normalized, but the coordinative aspects of movement were not. This led to the hypothesis that dopaminergic medication more readily compensates for intensive aspects of movement (such as speed), than for coordinative aspects (such as coordination of different limb segments) (Schettino et al., Exp Brain Res 168:186–202, 2006). We tested this hypothesis on rhythmic, continuous movements of the forearm. In our task, target peak speed and amplitude, availability of visual feedback, and medication state (on/off) were varied. We found, consistent with the discrete-movement results, that peak speed (intensive aspect) was normalized by medication, while accuracy, which required coordination of speed and amplitude modulation (coordinative aspect), was not normalized by dopaminergic treatment. However, our findings that amplitude, an intensive aspect of movement, was also not normalized by medication, suggests that a simple pathway gain increase does not act to remediate all intensive aspects of movement to the same extent. While it normalized movement peak speed, it did not normalize movement amplitude. Furthermore, we found that when visual feedback was not available, all participants (PD and controls) made faster movements. The effects of dopaminergic medication and availability of visual feedback on movement speed were additive. The finding that movement speed uniformly increased both in the PD and the control groups suggests that visual feedback may be necessary for calibration of peak speed, otherwise underestimated by the motor control system.







Similar content being viewed by others
Notes
UPDRS is a four-part rating scale. Part III of the UPDRS, whose score is reported here, is designed to assess severity of the motor symptoms in patients with PD. Each item is scored from 0 (normal) to 4 (maximal severity), for a maximum (worst) score of 108.
The minimum required peak speed was denoted on the screen by the vertical extent of the inner ellipse above (and below) the vertical midpoint.
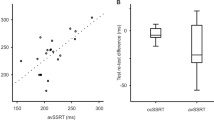
Note there may be a difference between the performance of more natural discrete reaching movements and that of the rhythmic task at hand.
Cf. Ghilardi et al. (2000).
References
Abbruzzese G, Berardelli A (2003) Sensorimotor integration in movement disorders. Mov Disord 18:231–240
Adamovich SV, Berkinblit MB, Hening W, Sage J, Poizner H (2001) The interaction of visual and proprioceptive inputs in pointing to actual and remembered targets in Parkinson’s disease. Neuroscience 104:1027–1041
Agostino R, Berardelli A, Formica A, Accornero N, Manfredi M (1992) Sequential arm movements in patients with Parkinson’s disease, Huntington’s disease, and dystonia. Brain 115(5):1481–1495
Almeida QJ, Wishart LR, Lee TD (2003) Disruptive influences of a cued voluntary shift on coordinated movement in Parkinson’s disease. Neuropsychologia 41:442–452
Almeida QJ, Frank JS, Roy EA, Jenkins ME, Spaulding S, Patla AE, Jog MS (2005) An evaluation of sensorimotor integration during locomotion toward a target in Parkinson’s disease. Neuroscience 134:283–293
Berardelli A, Dick JP, Rothwell JC, Day BL, Marsden CD (1986) Scaling of the size of the first agonist EMG burst during rapid wrist movements in patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry 49:1273–1279
Cardoso EF, Fregni F, Maia FM, Melo LM, Sato JR, Cruz Jr AC, Bianchi ET, Fernandes DB, Monteiro MLR, Barbosa ER (2010) Abnormal visual activation in Parkinson’s disease patients. Mov Disord 25(11):1590–1596
Chaput S, Proteau L (1996) Aging and motor control. J Gerontol Ser B Psychol Sci Soc Sci 51:346–355
Demirci M, Grill S, McShane L, Hallett M (1997) A mismatch between kinesthetic and visual perception in Parkinson’s disease. Ann Neurol 41:781–788
Doeringer JA, Hogan N (1998) Intermittency in preplanned elbow movements persists in the absence of visual feedback. J Neurophysiol 80:1787–1799
Farley BG, Koshland GF (2005) Training BIG to move faster: the application of the speed–amplitude relation as a rehabilitation strategy for people with Parkinson’s disease. Exp Brain Res 167:462–467
Fellows SJ, Noth J, Schwarz M (1998) Precision grip and Parkinson’s disease. Brain 121(Pt 9):1771–1784
Flash T, Inzelberg R, Schechtman E, Korczyn AD (1992) Kinematic analysis of upper limb trajectories in Parkinson’s disease. Exp Neurol 118:215–226
Flowers KA (1976) Visual ‘closed-loop’ and ‘open-loop’ characteristics of voluntary movement in patients with Parkinsonism and intention tremor. Brain 99:269–310
Flowers KA (1978) Lack of prediction in the motor behavior of Parkinsonism. Brain 101:35–52
Georgiou N, Iansek R, Bradshaw JL, Phillips JG, Mattingley JB, Bradshaw JA (1993) An evaluation of the role of internal cues in the pathogenesis of Parkinsonian hypokinesia. Brain 116:1575–1587
Ghilardi MF, Alberoni M, Rossi M, Franceschi M, Mariani C, Fazio F (2000) Visual feedback has differential effects on reaching movements in Parkinson’s and Alzheimer’s disease. Brain Res 876:112–123
Hallett M, Khoshbin S (1980) A physiological mechanism of bradykinesia. Brain 103:301–314
Hatsopoulos NG, Warren WH (1996) Resonance tuning in rhythmic arm movements. J Mot Behav 28:3–14
Hogan N, Sternad D (2007) On rhythmic and discrete movements: reflections, definitions and implications for motor control. Exp Brain Res 181:13–30
Holroyd S, Wooten GF (2006) Preliminary fMRI evidence of visual system dysfunction in Parkinson’s disease patients with visual hallucinations. J Neuropsychiatry Clin Neurosci 18:402–404
Inzelberg R, Korczyn AD (1996) Concerning” visual control of arm movement in Parkinson’s disease”. Mov Disord 11:115
Jackson SR, Jackson GM, Harrison J, Henderson L, Kennard C (1995) The internal control of action and Parkinson’s disease: a kinematic analysis of visually guided and memory-guided prehension movements. Exp Brain Res 105:147–162
Jacobs JV, Horak FB (2006) Abnormal proprioceptive-motor integration contributes to hypometric postural responses of subjects with Parkinson’s disease. Neuroscience 141:999–1009
Jobst EE, Melnick ME, Byl NN, Dowling GA, Aminoff MJ (1997) Sensory perception in Parkinson disease. Arch Neurol 54:450–454
Kelly VE, Hyngstrom AS, Rundle MM, Bastian AJ (2002) Interaction of levodopa and cues on voluntary reaching in Parkinson’s disease. Mov Disord 17:38–44
Klockgether T, Dichgans J (1994) Visual control of arm movement in Parkinson’s disease. Mov Disord 9:48–56
Klockgether T, Borutta M, Rapp H, Spieker S, Dichgans J (1995) A defect of kinesthesia in Parkinson’s disease. Mov Disord 10:460–465
Levy-Tzedek S, Krebs HI, Shils JL, Apetauerova D, Arle JE (2007) Parkinson’s disease: a motor control study using a wrist robot. Adv Robot 21:1201–1213
Levy-Tzedek S, Krebs H, Song D, Hogan N, Poizner H (2010) Non-monotonicity on a spatio-temporally defined cyclic task: evidence of two movement types? Exp Brain Res 202:733–746
Levy-Tzedek S, Ben Tov M, Karniel A (2011) Early switching between movement types: indication of predictive control? Brain Res Bull [Epub ahead of print]
Mink JW, Thach WT (1991) Basal ganglia motor control. I. Nonexclusive relation of pallidal discharge to five movement modes. J Neurophys 65:273–300
Morris ME, Iansek R, Matyas TA, Summers JJ (1996) Stride length regulation in Parkinson’s disease. Normalization strategies and underlying mechanisms. Brain 119(Pt 2):551–568
Pfann KD, Buchman AS, Comella CL, Corcos DM (2001) Control of movement distance in Parkinson’s disease. Mov Disord 16:1048–1065
Platz T, Brown RG, Marsden CD (1998) Training improves the speed of aimed movements in Parkinson’s disease. Brain 121:505–514
Poizner H, Feldman A, Levin M, Berkinblit M, Hening W, Patel A, Adamovich S (2000) Arm-trunk coordination is deficient and vision-dependent in Parkinson’s patients during reaching movements. Exp Brain Res 133:279–292
Raftery A, Cusumano J, Sternad D (2008) Chaotic frequency scaling in a coupled oscillator model for free rhythmic actions. Neural Comput 20:205–226
Rowe JB, Hughes L, Ghosh BCP, Eckstein D, Williams-Gray CH, Fallon S, Barker RA, Owen AM (2008) Parkinson’s disease and dopaminergic therapy—differential effects on movement, reward and cognition. Brain 131:2094–2105
Schaal S, Sternad D, Osu R, Kawato M (2004) Rhythmic arm movement is not discrete. Nat Neurosci 7(10):1136–1143
Schettino LF, Adamovich SV, Hening W, Tunik E, Sage J, Poizner H (2006) Hand preshaping in Parkinson’s disease: effects of visual feedback and medication state. Exp Brain Res 168:186–202
Schneider JS, Diamond SG, Markham CH (1986) Deficits in orofacial sensorimotor function in Parkinson’s disease. Ann Neurol 19:275–282
Sheridan MR, Flowers KA (1990) Movement variability and bradykinesia in Parkinson’s disease. Brain 113:1149–1161
Siegert RJ, Harper DN, Cameron FB, Abernethy D (2002) Self-initiated versus externally cued reaction times in Parkinson’s disease. J Clin Exp Neuropsychol 24:146–153
Silva MF, Faria P, Regateiro FS, Forjaz V, Januario C, Freire A, Castelo-Branco M (2005) Independent patterns of damage within magno-, parvo-and koniocellular pathways in Parkinson’s disease. Brain 128:2260
Tatton WG, Eastman MJ, Bedingham W, Verrier MC, Bruce IC (1984) Defective utilization of sensory input as the basis for bradykinesia, rigidity and decreased movement repertoire in Parkinson’s disease: a hypothesis. Can J Neurol Sci 11:136–143
Tunik E, Feldman AG, Poizner H (2007) Dopamine replacement therapy does not restore the ability of Parkinsonian patients to make rapid adjustments in motor strategies according to changing sensorimotor contexts. Parkinsonism Relat Disord 13:425–433
Yu H, Sternad D, Corcos DM, Vaillancourt DE (2007) Role of hyperactive cerebellum and motor cortex in Parkinson’s Disease. NeuroImage 35:222–233
Acknowledgments
This research was supported in part by National Institutes of Health (NIH) grants #2 R01 NS036449-11 and 1 R01-HD045343. SL was supported in part by a Howard Hughes Medical Institute Fellowship and the VA Veterans Affairs Grant B3607R at MIT and is currently funded by a fellowship from The Edmond and Lily Safra Center for Brain Sciences at The Hebrew University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Levy-Tzedek, S., Krebs, H.I., Arle, J.E. et al. Rhythmic movement in Parkinson’s disease: effects of visual feedback and medication state. Exp Brain Res 211, 277–286 (2011). https://doi.org/10.1007/s00221-011-2685-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-011-2685-0