Abstract
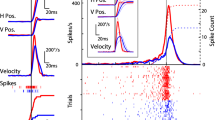
The smooth-pursuit system moves the eyes in space accurately to track slowly moving objects of interest despite visual inputs from the moving background and/or vestibular inputs during head movements. Recently, our laboratory has shown that young primates exhibit asymmetric eye movements during vertical pursuit across a textured background; upward eye velocity gain is reduced. To further understand the nature of this asymmetry, we performed three series of experiments in young monkeys. In Experiment 1, we examined whether this asymmetry was due to an un-compensated downward optokinetic reflex induced by the textured background as it moves across the retina in the opposite direction of the pursuit eye movements. For this, we examined the monkeys’ ability to fixate a stationary spot in space during movement of the textured background and compared it with vertical pursuit across the stationary textured background. We also examined gains of optokinetic eye movements induced by downward motion of the textured background during upward pursuit. In both task conditions, gains of downward eye velocity induced by the textured background were too small to explain reduced upward eye velocity gains. In Experiment 2, we examined whether the frame of reference for low-velocity, upward pursuit was orbital or earth vertical. To test this, we first applied static tilt in the roll plane until the animals were nearly positioned on their side in order to dissociate vertical or horizontal eye movements in the orbit from those in space. Deficits were observed for upward pursuit in the orbit but not in space. In Experiment 3, we tested whether asymmetry was observed during head-free pursuit that requires coordination between eye and head movements. Asymmetry in vertical eye velocity gains was still observed during head-free pursuit although it was not observed in vertical head velocity. These results, taken together, suggest that the asymmetric eye movements during vertical pursuit are specific for upward, primarily eye pursuit in the orbit.








Similar content being viewed by others
References
Akao T, Kurkin S, Fukushima J, Fukushima K (2005) Visual and vergence eye movement-related responses of pursuit neurons in the caudal frontal eye fields to motion-in-depth stimuli. Exp Brain Res 164:92–108
Bremmer F, Klam F, Duhamel JR, Ben Hamed S, Graf W (2002) Visual-vestibular interactive responses in the macaque ventral intraparietal area (VIP). Eur J Neurosci 16:1569–1586
Brettler SC, Baker J F (2003) Timing of low frequency responses of anterior and posterior canal vestibulo-ocular neurons in alert cats. Exp Brain Res 149:167–173
Chubb MC, Fuchs A F (1982) Contribution of y group of vestibular nuclei and dentate nucleus of cerebellum to generation of vertical smooth eye movements. J Neurophysiol 48:75–99
Cohen B, Matsuo V, Raphan T (1977) Quantitative analysis of the velocity characteristics of optokinetic nystagmus and optokinetic after-nystagmus. J Physiol (Lond) 270:321–344
Colby CL, Duhamel JR, Goldberg ME (1993) Ventral intraparietal area of the macaque: anatomic location and visual response properties. J Neurophysiol 69:902–914
Collewijn H, Conjin P, Tamminga EP (1982) Eye-head coordination in man during the pursuit of moving targets. In: Lennerstrand G, Zee DS, Keller EL (eds) Functional basis of ocular motility disorders. Pergamon, Oxford, pp 369–385
Fuchs AF, Robinson DA (1966) A method for measuring horizontal and vertical eye movements chronically in the monkey. J Appl Physiol 21:1068–1070
Fukushima J, Akao T, Takeichi N, Kaneko CRS, Fukushima K (2003) Involvement of the frontal oculomotor areas in developmental compensation for the directional asymmetry in smooth pursuit eye movements in young primates. Ann NY Acad Sci 1004:451–456
Fukushima J, Akao T, Takeichi N, Kurkin S, Kaneko CR, Fukushima K (2004) Pursuit-related neurons in the supplementary eye fields: discharge during pursuit and passive whole body rotation. J Neurophysiol 91:2809–2825
Fukushima K (2003) Frontal cortical control of smooth-pursuit. Curr Opin Neurobiol 13:647–654
Fukushima K, Fukushima J, Kaneko CRS, Fuchs AF (1999) Vertical Purkinje cells of the monkey floccular lobe: simple-spike activity during pursuit and passive whole body rotation. J Neurophysiol 82:787–803
Fukushima K, Sato T, Fukushima J, Shinmei Y, Kaneko CR (2000) Activity of smooth pursuit-related neurons in the monkey periarcuate cortex during pursuit and passive whole-body rotation. J Neurophysiol 83:563–587
Fukushima K, Yamanobe T, Shinmei Y, Fukushima J, Kurkin S, Peterson BW (2002) Coding of smooth eye movements in three-dimensional space by frontal cortex. Nature 419:157–162
Grasse KL, Lisberger SG (1992) Analysis of a naturally occurring asymmetry in vertical smooth pursuit eye movements in a monkey. J Neurophysiol 67:164–179
Heinen SJ, Liu M (1997) Single-neuron activity in the dorsomedial frontal cortex during smooth-pursuit eye movements to predictable target motion. Visual Neurosci 14:853–865
Hirai N, Uchino Y (1984) Floccular influence on excitatory relay neurones of vestibular reflexes of anterior semicircular canal origin in the cat. Neurosci Res 1:327–340
Ito M, Nishimaru N,Yamamoto M (1977) Specific patterns of neuronal connections involved in the control of the rabbit’s vestibulo-ocular reflexes by the cerebellar flocculus. J Physiol 265:833–854
Kaneko CRS, Fukushima K (1998) Discharge characteristics of vestibular saccade neurons in alert monkeys. J Neurophysiol 79:835–847
Kawano K, Miles FA (1986) Short-latency ocular following responses of monkey. II. Dependence on a prior saccadic eye movement. J Neurophysiol 56:1355–1380
Krauzlis RJ, Lisberger SG (1996) Directional organization of eye movement and visual signals in the floccular lobe of the monkey cerebellum. Exp Brain Res 109:289–302
Leigh RJ, Zee DS (1999) The neurology of eye movements. 3rd edn. Oxford University Press, New York, pp 4–197
Lisberger SG, Fuchs AF (1978) Role of primate flocculus during rapid behavioral modification of vestibuloocular reflex. I. Purkinje cell activity during visually guided horizontal smooth-pursuit eye movements and passive head rotation. J Neurophysiol 41:733–763
Lisberger SG, Miles FA, Optican LM, Eighmy BB (1981) Optokinetic response in monkey: underlying mechanisms and their sensitivity to long-term adaptive changes in vestibuloocular reflex. J Neurophysiol 45:869–890
Lisberger SG, PavelkoTA, Bronte-Stewart HM, Stone LS (1994) Neural basis for motor learning in the vestibuloocular reflex of primates. II. Changes in the responses of horizontal gaze velocity Purkinje cells in the cerebellar flocculus and ventral paraflocculus. J Neurophysiol 72:954–973
MacAvoy MG, Gottlieb JP, Bruce CJ (1991) Smooth pursuit eye movement representation in the primate frontal eye field. Cerebral Cortex 1:95–102
Matsuo V, Cohen B (1984) Vertical optokinetic nystagmus and vestibular nystagmus in the monkey: up-down asymmetry and effects of gravity. Exp Brain Res 53:197–216
Miles FA, Fuller JH, Braitman DJ, Dow BM (1980) Long-term adaptive changes in primate vestibuloocular reflex. III. Electrophysiological observations in flocculus of normal monkeys. J Neurophysiol 43:1437–1476
Mouri T (1994) Postnatal growth and sexual dimorphism in the skull of the Japanese macaque (Macaca fuscata). Anthropol Sci 102(Suppl):43–56
Nagao S, Kitamura T, Nakamura N, Hiramatsu T, Yamada J (1997) Location of efferent terminals of the primate flocculus and ventral paraflocculus revealed by anterograde axonal transport methods. Neurosci Res 27:257–269
Sakata H, Shibutani H, Kawano K (1983) Functional properties of visual tracking neurons in posterior parietal association cortex of the monkey. J Neurophysiol 49:1364–1380
Sato U, Kawasaki T (1990) Operational unit responsible for plane-specific control of eye movement by cerebellar flocculus in cat. J Neurophysiol 64:551–564
Scudder CA, Fuchs AF (1992) Physiological and behavioral identification of vestibular nucleus neurons mediating the horizontal vestibuloocular reflex in trained rhesus monkeys. J Neurophysiol 68:244–264
Shidara M, Kawano K (1993) Role of Purkinje cells in the ventral paraflocculus in short-latency ocular following responses. Exp Brain Res 93:185–195
Shinmei Y, Yamanobe T, Fukushima J, Fukushima K (2002) Purkinje cells of the cerebellar dorsal vermis in the monkey: simple-spike activity during pursuit and passive whole body rotation. J Neurophysiol 87:1836–1849
Singh A, Thau GE, Raphan T, Cohen B (1981) Detection of saccades by a maximum likelihood ratio criterion. In: Proceedings of the 34th Annual Conference Eng Biol, Houston, p 136
Stone LS, Lisberger SG (1990) Visual responses of Purkinje cells in the cerebellar flocculus during smooth-pursuit eye movements in monkeys. I. Simple spikes. J Neurophysiol 63:1241–1261
Takeichi N, Fukushima J, Kurkin S, Yamanobe T, Shinmei Y, Fukushima K (2003) Directional asymmetry in smooth ocular tracking in the presence of visual background in young and adult primates. Exp Brain Res 149:380–390
Tsubuku T, Akao T, McCrea RA, Kurkin SA, Fukushima J, Fukushima K (2004) Purkinje cell activity in the cerebellar floccular region during vergence eye movements. Soc Neurosci (Abstr) Program No. 880.3. Abstract Viewer/Itinerary Planner. San Diego
Zhang Y, Partsalis AM, Highstein SM (1995) Properties of superior vestibular nucleus flocculus target neurons in the squirrel monkey. I. General properties in comparison with flocculus projecting neurons. J Neurophysiol 73:2261–2278
Acknowledgements
We thank Dr. C.R.S. Kaneko for his valuable comments on this manuscript. This research was supported by Grant-in-Aid for Scientific Research on Priority Areas (System study on higher-order brain functions) from the Ministry of Education, Culture, Sports, Science and Technology of Japan (17022001), Marna Cosmetics, and Toyota Riken
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kasahara, S., Akao, T., Fukushima, J. et al. Further evidence for selective difficulty of upward eye pursuit in juvenile monkeys: effects of optokinetic stimulation, static roll tilt, and active head movements. Exp Brain Res 171, 306–321 (2006). https://doi.org/10.1007/s00221-005-0278-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-005-0278-5