Abstract
Interspecies hybridization has been shown to be a powerful tool for developing and improving brewing yeast in a number of industry-relevant respects. Thanks to the popularity of heavily hopped ‘India Pale Ale’-style beers, there is an increased demand from brewers for strains that can boost hop aroma. Here, we explored whether hybridization could be used to construct strains with an enhanced ability to release hop-derived flavours through β-lyase activity, which releases desirable volatile thiols. Wild Saccharomyces strains were shown to possess high β-lyase activity compared to brewing strains, however, they also produced phenolic off-flavours (POF) and showed poor attenuation. To overcome these limitations, interspecies hybrids were constructed by crossing pairs of one of three brewing and one of three wild Saccharomyces strains (S. uvarum and S. eubayanus). Hybrids were screened for fermentation ability and β-lyase activity, and selected hybrids showed improved fermentation and formation of both volatile thiols (4MMP, 3MH and 3MH-acetate) and aroma-active esters compared to the parent strains. Undesirable traits (e.g. POF) could be removed from the hybrid by sporulation. To conclude, it was possible to boost the release of desirable hop-derived thiols in brewing yeast by hybridization with wild yeast. This allows production of beer with boosted hop aroma with less hops (thus improving sustainability issues).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The interest towards heavily hopped ale beer and beer with novel and diverse flavours has increased considerably the past years [1,2,3]. For instance, hop production and consumption within the brewery in the USA has grown markedly in the past decade [4, 5]. Interest towards the use of atypical yeast strains as a strategy for diversifying flavour has also grown [6,7,8]. Indeed, beer flavour varies considerably depending on the brewing yeast strain employed for fermentation. However, the different flavour profiles tend to vary in a quantitative rather than qualitative way, with brewing yeast producing similar flavour compounds (e.g. higher alcohols, acetate esters, and ethyl esters), albeit in different quantities. To diversify and introduce new flavours, as well as meet the demand for enhanced hop flavour, brewers have begun exploiting the ability of yeast to enzymatically alter hop-derived compounds in the beer [9,10,11]. This process is often referred to as ‘biotransformation’, and the term encompasses many different types of reactions. Among the key biotransformation reactions are those catalysed by β-lyase enzymes. These enzymes can liberate volatile thiols from their conjugated (glutathionylated or cysteinylated) and therefore non-active forms [12,13,14]. Volatile thiols, such as 4-mercapto-4-methyl-2-pentanone (4MMP), 3-mercapto-1-hexanol (3MH), and 3-mercaptohexylacetate (3MHA), have been shown to have a vital role in modern hop aroma. These compounds contribute desirable tones of black currant, grapefruit, and passion fruit, and have extremely low flavour thresholds, often in the ng/L range [15].
While some hop varieties may directly contribute considerable amounts of free thiols, they, together with malt, have also been shown to contain high concentrations of conjugated 3MH and 4MMP precursors [12]. Indeed, the vast majority of all thiols in hops are present in cysteine- or glutathione-conjugated form and are, therefore, odourless [12]. Hence, there exists a large potential pool of flavour that can be unmasked through the use of yeast expressing the β-lyase activity. This would allow for the production of beer with detectable thiol levels using hop varieties that are otherwise low in free thiols (e.g. Perle or Cascade; Roland et al. 2016). However, β-lyase activity varies considerably among brewing strains because of widespread inactivating mutations in the key β-lyase-encoding gene IRC7 [16,17,18]. When such activities are seen in wine or beer fermentations, they are typically associated with wild strains [18, 19]. The β-lyase activity of Saccharomyces species outside of S. cerevisiae has not been explored much. From limited studies, it appears as if Saccharomyces uvarum strains tend to have higher thiol release abilities than S. cerevisiae strains [20, 21]. Interspecific hybridization between S. cerevisiae and S. uvarum strains has also been shown to enhance thiol release during wine fermentations in comparison to using S. cerevisiae by itself [21, 22]. A possible explanation for the enhanced β-lyase activity of S. uvarum is that IRC7 is located further away from the telomeres in S. uvarum compared to S. cerevisiae, and, therefore, less likely to be downregulated by sub-telomeric silencing as in S. cerevisiae [23].
S. uvarum is not traditionally used in beer fermentation, but is associated with wine and cider fermentations. Its close ancestor, Saccharomyces eubayanus, is also not traditionally used in beer fermentations, however, the species has formed an interspecies hybrid with S. cerevisiae to give rise to lager yeast [24]. Recent studies have shown that both species do, however, have some brewing potential, as strains of both species tend to be able to utilise the main wort sugar maltose [25,26,27,28]. They are not capable of using all fermentable sugars though, and also possess other negative qualities from a brewing perspective, in particular the production of phenolic flavours. Studies have shown that these wild-yeast deficiencies can be overcome through yeast breeding [25, 29,30,31]. As mentioned above, breeding or hybridization has also been used to construct yeast strains with enhanced β-lyase activities [16, 32,33,34]. There is potential to create hybrid strains (S. cerevisiae × S. uvarum or S. cerevisiae × S. eubayanus) that incorporate the normal brewing yeast capabilities as well as an enhanced ability to release hop-derived flavours through enzymatic activity. Such strains could not only allow the introduction of new and enhanced flavour to beer, but also potentially yield cost and environmental impact savings from the need to use less hops to produce the same amount of flavour.
Here, we first screened the β-lyase activity of a set of potential parent strains. We then constructed interspecies hybrids between strains of S. cerevisiae and either S. uvarum or S. eubayanus using rare mating. Hybrids were then screened for fermentation ability and β-lyase activity. The hybrid with most efficient thiol release was phenolic off-flavour positive (POF+), so it was further mated with a POF− S. cerevisiae parent strain to form a three-parent hybrid. This hybrid was sporulated and POF− spore clones were successfully obtained. The fermentation performance and ability to release volatile thiols of this spore clone was demonstrated at 10 L-scale. Our study demonstrates how the release of desirable hop-derived thiols in brewing yeast can be boosted by hybridization with wild yeast.
Materials and methods
Yeast strains
Lists of all yeast strains included in this study are found in Tables 1 and 2. Table 1 contains a list of the included parent and control strains, while Table 2 contains a list of all constructed hybrid strains.
Hybrid construction
Interspecific hybrids between brewing and wild Saccharomyces strains were generated using both spore-to-spore mating and rare mating. Prior to rare mating, natural auxotrophic mutants (lys − or ura −) of the parental strains were selected on α-aminoadipic and 5-fluoroorotic acid agar plates, respectively [35, 36]. Auxotrophy was confirmed by the inability to grow on minimal selection agar medium (0.67% Yeast Nitrogen Base without amino acids, 3% glycerol, 3% ethanol and 2% agar).
For spore-to-spore mating, ascospores of the parental strains were generated by plating on 1% potassium acetate agar. Ascus walls were digested with 1 mg mL−1 Zymolyase 100 T (US Biological, USA), after which spores from the different parental strains were dissected and placed next to each other on YPD agar plates (1% yeast extract, 2% peptone, 2% glucose, and 2% agar) using a micromanipulator (Singer Instruments, UK). The plates were incubated at 25 °C for 3 days, after which any emerging colonies were replated on minimal selection agar, and incubated at 25 °C for 5 days. Any colonies emerging on the minimal selection agar were regarded as potential hybrids.
For rare mating, cultures of parent strains with complementary auxotrophy were grown overnight at 25 °C by inoculating a single colony into 50 mL of YPM (1% yeast extract, 2% peptone, 2% maltose). One hundred microliters of the resulting overnight yeast cultures from both parental strains was transferred together to 1 mL YPM medium in a sterile 2 mL Eppendorf tube. Tubes were vortexed and incubated statically at 25 °C for 5 days. After incubation, the tubes were centrifuged at 5000g for 5 min and the supernatant was removed. Five hundred microliters of starvation medium (0.1% yeast extract and 0.1% glucose) was added, and tubes were incubated for at least 2 h at room temperature. Tubes were then vortexed and 100 μL aliquots were spread onto minimal selection agar (without uracil or lysine). Plates were incubated at 25 °C for 5 days and any colonies emerging on the minimal selection agar were regarded as potential hybrids.
The three-parent hybrid D2 was constructed through spore-to-spore mating using S. cerevisiae A132 and hybrid A62 × C1037 L2 as parent strains.
Selected hybrids were transferred to 1% potassium acetate agar for sporulation in an attempt to remove the POF phenotype. After 7 days of incubation at 25 °C, ascospores were digested using Zymolyase 100 T (US Biological, Salem, MA, USA) and dissected on YPD agar using the MSM400 dissection microscope (Singer Instruments, Roadwater, UK).
Hybrid confirmation
The hybrid status of potential hybrids was confirmed by PCR as described in Krogerus et al. (2015). Briefly, the rDNA ITS region was amplified using the primers ITS1 (5′-TCCGTAGGTGAACCTGCGG-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′), and amplicons were digested using the HaeIII restriction enzyme (New England BioLabs, USA) as described previously [37]. The hybrid status of the potential three-parent hybrids was confirmed by PCR using delta12 (5′-TCAACAATGGAATCCCAAC-3′) and delta21 (5′-CATCTTAACACCGTATATGA-3′) primers.
Enzyme activity of yeasts
β-Lyase activity was estimated by measuring growth on cysteine. Media contained 0.17% Yeast Nitrogen Base without (NH4)2SO4 and amino acids, 1% glucose, 0.01% pyridoxal 5-phosphate, and 15 mM of cysteine. Growth assays were carried out in 96-well plates, with 145 μL media per well. Wells were inoculated (to a starting OD600 value of 0.1) with 5 µL of washed pre-culture suspended in water to an OD600 value of 3. Plates were sealed with a Breathe-Easy membrane (Sigma-Aldrich, Espoo, Finland), and incubated at 25 °C for one week. OD600 values were measured on a VarioSkan plate reader (Thermo Scientific, Vantaa, Finland), while cysteine content of the growth media was estimated using DTNB [38].
The ability to produce phenolic off-flavour was estimated using the absorbance-based method described by Mertens et al. [39].
Wort fermentations
Lab-scale wort fermentations were first carried out to screen the yeast hybrids in order to identify top-performing strains that were able to rapidly attenuate wort sugars. Fermentations were carried out in 100 mL Schott bottles capped with glycerol-filled airlocks. Yeast strains were grown overnight in 25 mL YPM medium at 25 °C. The pre-cultured yeast was then inoculated into 80 mL of all-malt wort (extract content of 15°Plato) at a rate of 5 g fresh yeast L−1. Fermentations were carried out in duplicate at 25 °C for 7 days. Fermentations were monitored by mass lost as CO2.
2 L- and 10 L-scale wort fermentations were carried out in 3 L and 12 L cylindroconical stainless steel fermenting vessels, containing 2 L of 15°P wort or 10 L of 12°P wort. Yeast was propagated in autoclaved wort. The worts were produced at the VTT Pilot Brewery from barley malt and contained 2.5 g L−1 each of Cascade and Perle hops added to the whirlpool. The wort was oxygenated to 10 mg L−1 prior to pitching (Oxygen Indicator Model 26073 and Sensor 21158; Orbisphere Laboratories, Geneva, Switzerland). Yeast was inoculated at a rate of 106 viable cells mL−1°Plato−1, together with 5 g L−1 of Cascade hops (dry hopping). The fermentations were carried out in triplicate at 18 °C until no change in alcohol level was observed for 24 h or for a maximum of 9 days.
Wort samples were drawn regularly from the fermentation vessels aseptically and placed directly on ice, after which the yeast was separated from the fermenting wort by centrifugation (9000g, 10 min, 1 °C).
Beer chemical analysis
The specific gravity, alcohol level (% v/v), and pH of samples were determined from the centrifuged and degassed fermentation samples using an Anton Paar Density Metre DMA 5000 M with Alcolyzer Beer ME and pH ME modules (Anton Paar GmbH, Graz, Austria).
Concentrations of fermentable sugars (glucose, fructose, maltose, and maltotriose) and ethanol were measured by HPLC using a Waters 2695 Separation Module and Waters System Interphase Module liquid chromatograph coupled with a Waters 2414 differential refractometer (Waters Co., Milford, MA, USA). An Aminex HPX-87H Organic Acid Analysis Column (300 × 7.8 mm; Bio-Rad, Hercules, CA, USA) was equilibrated with 5 mM H2SO4 (Titrisol, Merck, Darmstadt, Germany) in water at 55 °C, and samples were eluted with 5 mM H2SO4 in water at a 0.3 mL min−1 flow rate.
Higher alcohols, esters and 4-vinyl guaiacol concentrations were analysed using headspace solid-phase micro-extraction coupled with gas chromatography (Agilent 7890A)—mass spectrometry (Agilent 5975C; HS-SPME–GC–MS) by modifying the method used by Rodriguez-Bencomo et al. [40]. 4 mL of beer sample, 1.8 g of NaCl, and 50 µL of internal standard solution (containing 1.28 µg 3-octanol, 1.19 µg 3,4-dimethylphenol) were added to 20 mL headspace vials. The samples were pre-incubated in Gerstel MPS autosampler at 44.8 °C for 10 min and the volatiles were extracted using a 2 cm divinylbenzene/carboxen/polydimethylsiloxane (DVB/CAR/PDMS) fibre (Supelco) at 44.8 °C for 46.8 min. The samples were injected in splitless mode (10 min desorption time at 270 °C) and the compounds were separated on an HP-Innowax silica capillary column (60 m, 0.250 mm i.d., 0.25 µm film thickness). The oven temperature program was from 40 °C (3 min) to 240 °C (4 °C min−1) and the final temperature was held for 15 min. The MS data were collected at a mass range of 35–450 amu. Identification was based on spectral data of reference compounds and those of NIST 08 library. Calibration curves determined for 2-methoxy-4-vinylphenol, 2-phenylethyl acetate, 3-methylbutyl acetate, and ethyl esters of acetic, butyric, decanoic, hexanoic and octanoic acids, respectively (r2 = 0.933–0.999).
The volatile thiols 4-mercapto-4-methyl-2-pentanone (4MMP), 3-mercapto-1-hexanol (3MH), and 3-mercaptohexylacetate (3MHA) were determined at VLB Berlin using the method described by Dennenlöhr et al. [50]. In this method, thiols are extracted and derivatized by headspace solid-phase micro-extraction (HS-SPME) with on-fiber derivatization (OFD) using 2,3,4,5,6-pentafluorobenzyl bromide (PFBBr). Resulting PFBBr-thioesters are then separated and analysed using gas chromatography tandem mass spectrometry (GC–MS/MS). The instrumental setup, parameters of sample preparation, GC–MS/MS analysis, calibration, and quantification were in accordance to Dennenlöhr et al. [50], with an exception that the analysis was performed on an Agilent 7010B triple quadrupole MS with high-efficiency source instead of an Agilent 7000C triple quadrupole MS. Each sample was analysed in duplicate.
Whole-genome sequencing and analysis
S. cerevisiae A132, S. uvarum C1037 and three hybrid strains were whole-genome sequenced in-house. DNA was extracted using the method described by Denis et al. [41]. Sequencing was carried out on an Oxford Nanopore MinION MK1C instrument on a R10.3 flow cell using the SQK-LSK110 ligation sequencing kit. Reads were basecalled using Guppy version 5.0.11 using the ‘super high accuracy’ model. Basecalling statistics are available in Supplementary Table S1. Genomes of S. cerevisiae A132 and S. uvarum C1037 were de novo assembled using Flye version 2.9 [42]. Assemblies were polished using Medaka version 1.4.3. Reads from the hybrid strains were aligned to a concatenated reference genome of S. cerevisiae A81062 [43] and S. uvarum C1037 using minimap2 version 2.17-r941 [44]. Sequencing coverage was estimated with mosdepth (version 0.2.6; Pedersen and Quinlan [45]). Variants were called with clair3 [46]. Basecalled reads have been deposited in NCBI-SRA under BioProject number PRJNA820581.
Results
Screening β-lyase activity in parent strains
As the objective of the study was to construct brewing yeast strains with enhanced ability to release volatile thiols from wort, we first screened the β-lyase activity in a range of Saccharomyces strains. The set included S. pastorianus lager strains, S. cerevisiae ale strains, as well as S. eubayanus and S. uvarum wild strains. β-Lyase activity was estimated based on the ability to grow on and consume 15 mM cysteine as the sole nitrogen source. This requires expression of a functional β-lyase-encoding IRC7 gene in S. cerevisiae, and correlates with ability to release 4MMP from its cysteinylated form [16, 18, 47]. The strains showed variation in ability to grow and consume cysteine, with the S. uvarum strains showing the highest growth and consumption (Fig. 1A, B). The poorest growth was observed for the lager and selected ale strain, while S. cerevisiae A60, S. cerevisiae A62 and S. eubayanus C961 showed intermediate growth and consumption. A strong positive correlation was observed between growth on 15 mM cysteine and its consumption (Fig. 1C). Results suggest that hybridization of ale strains with either of the S. uvarum strains show highest potential for generating a hybrid strain with both efficient fermentation and ability to release volatile thiols.
The ability to A grow on 15 mM cysteine as a sole nitrogen source and B consume cysteine (% of original amount) during cultivations. C The correlation and Pearson correlation coefficient (r) between growth and cysteine consumption. Strains are coloured based on type: blue, lager; red, ale; orange, wild. Different letters above bars indicate significant differences between values as determined by one-way ANOVA and Tukey’s post hoc test (p < 0.05)
Construction of interspecific hybrids
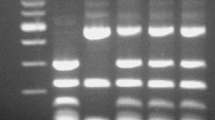
Following the initial screening, we attempted to construct interspecies hybrids between one of three S. cerevisiae ale strains (A60, A62 and A132) and one of three wild Saccharomyces strains (S. uvarum BFPBL32, S. uvarum C1037 and S. eubayanus C961). Hybrid construction was first attempted with rare mating, to ensure the whole genome of both parent strains were to be retained. As rare mating requires selection markers for the parent strains, we selected auxotrophic or petite mutants of the parent strains prior to mating. Uracil auxotrophs of S. cerevisiae A62 and A132, a petite mutant of S. cerevisiae A60, as well as lysine auxotrophs of S. eubayanus C961 and S. uvarum C1037 were successfully obtained. No auxotrophs of S. uvarum BFPBL32 were obtained, and the strain had to be excluded from the rare mating attempts. Rare mating resulted in ten successful hybrids of four different hybrid combinations (see Table 1). Hybrid status was confirmed using PCR, showing presence of genetic material from both parent strains (Fig. 2).
Confirmation of hybridization by A rDNA ITS PCR and RFLP, and B amplification of DBP6 and MEX67 genes. Lane 1 is a 100 bp DNA ladder, lane 2 S. cerevisiae A62, lane 3 S. uvarum C1037 and lane 4 an interspecies hybrid between the two. C The ability to grow on 15 mM cysteine as a sole nitrogen source. Strains are coloured based on type: blue, hybrid; red, ale; orange, wild. Different letters above bars indicate significant differences between values as determined by one-way ANOVA and Tukey’s post hoc test (p < 0.05)
As not all hybrid combination were successful, it was further attempted to construct hybrids using spore-to-spore mating. This technique does not require selection of auxotrophic mutants of the parent strains prior to mating, but the parent strains need to be able to produce viable spores. Of the three brewing strains, only S. cerevisiae A132 sporulated efficiently. S. uvarum C1037 also did not sporulate. As hybrids between A132 and C961 were already obtained with rare mating, spore-to-spore mating was ultimately only tested between S. cerevisiae A132 and S. uvarum BFPBL32. Two successful hybrids were obtained (see Table 1).
Hybrid screening
Following successful construction of interspecific hybrids, the 12 hybrid strains were screened for β-lyase activity in the same way as the parent strains. Variation between the hybrids was observed, and strains tended to exhibit mid-parent heterosis (values in between parent strains) in regards to ability to grow on 15 mM cysteine as a sole nitrogen source (Fig. 2C). The highest growth among the hybrids was observed for the A62 × C1037 L2.
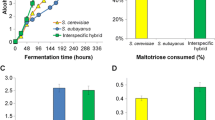
After screening β-lyase activity, 80 mL-scale 15°Plato wort fermentations were carried out with 8 hybrids and the 6 parent strains to identify hybrids with good fermentation performance. Most hybrids had fermentation rates in between those of the parent strains, but certain hybrids outperformed both parent strains (Fig. 3A–C). These include the A60 × C961 hybrid and several hybrids created with S. cerevisiae A62. Similar results, of hybrids outperforming the parent strains in regards to fermentation, have been observed before in multiple hybrid combinations [16, 25, 29, 48]. However, on average, there was no significant difference between the final attenuation reached by the parents or hybrids (Fig. 3D). Two hybrids were selected for 2 L-scale fermentations based on fermentation performance and β-lyase activity.
The fermentation performance (% mass lost as CO2) of de novo hybrids compared to parent strains during 80 mL-scale fermentation with 15°Plato wort. A–C Strains are plotted in separate graphs based on the S. cerevisiae brewing parent: A S. cerevisiae A60, B S. cerevisiae A62, and C S. cerevisiae A132. D The average time (hours) until 50% of final attenuation was reached in the three strain groups. Strains are coloured based on type: blue, hybrid; red, ale; orange, wild
Wort fermentations confirm enhanced phenotypes in hybrids
2 L-scale fermentations in 15°Plato wort were performed with the two selected hybrids (A62 × C1037 L2 and A132 × BFPBL32 C1) and the four parent strains that were used for their creation. Fermentation performance at 2 L-scale was similar to that during the smaller scale fermentations (Fig. 4A, B). One hybrid (A62 × C1037 L2) fermented faster than both parent strains, while the other fermented slower than both parent strains (A132 × BFPBL32 C1). Of the hybrids, only A62 × C1037 L2 was able to consume maltotriose. This trait was inherited from S. cerevisiae A62, which was also the only parent strain capable of maltotriose use.
The fermentation performance of de novo hybrids compared to parent strains during 2 L-scale fermentation with 15°Plato wort. A, B The alcohol by volume (%) produced during fermentation by A Hybrid A62 × C1037 L2 and B Hybrid A132 × BFPBL32 C1 compared to their respective parent strains. The concentrations (ng/L) of C 4-mercapto-4-methyl-2-pentanone (4MMP), D 3-mercapto-1-hexanol (3MH), and E 3-mercaptohexyl acetate (3MHA) in the resulting beers. The concentrations (mg/L) of (F) 3-methylbutyl acetate and G ethyl hexanoate in the resulting beers. Curves and bars are coloured based on type: blue, hybrid; red, ale; orange, wild. Different letters above bars indicate significant differences between values as determined by one-way ANOVA and Tukey’s post hoc test (p < 0.05)
The beers showed significant variation in concentrations of volatile thiols (Fig. 4C, E). Interestingly, the hybrids tended to show either higher or as high concentrations of 4MMP, 3MH and 3MH-acetate compared to the better parent (i.e. the wild Saccharomyces strain). Thiol levels had increased many fold from those measured in the wort, indicating β-lyase activity during fermentation (data not shown). Concentrations of 4MMP and 3MH were in almost all cases above the flavour threshold of 1–1.5 ng/L and 55–60 ng/L, respectively [15, 49]. The A62 × C1037 hybrid, which had exhibited the most efficient fermentation, also produced beer with the highest levels of volatile thiols. Hence, the objective of creating a strain with both efficient fermentation and an enhanced ability to release hop-derived thiols was successfully reached.
The beers also varied in their aroma-active ester content (Fig. 4F, G). The A62 × C1037 hybrid again exhibited best-parent heterosis and produced higher levels of esters compared to either parent. For the A132 × BFPBL32 hybrid, concentrations of 3-methylbutyl acetate and ethyl hexanoate were lower than and in between the parents, respectively. The beer made with the A62 × C1037 hybrid had the highest levels of volatile thiols, 3-methylbutyl acetate and ethyl hexanoate, and, therefore, appeared to produce the most fruity and complex aroma. However, as both parent strains of this hybrid were POF+, the hybrid was also POF+. This trait is undesired in most beer styles, and we thus next attempted to remove the phenotype.
Construction of three-parent hybrid and POF− spore clone
To further develop the most promising, but POF+, hybrid A62 × C1037, an attempt was made to mate the hybrid with a POF− S. cerevisiae A132 parent strain. This was accomplished through spore-to-spore mating. As the hybrid was tetraploid (being a result of rare mating), it was able to sporulate and produce viable spores despite being an interspecies hybrid. These spores were crossed with spores of S. cerevisiae A132 to form a three-parent hybrid. Hybrid status was assessed using interdelta PCR, and a single confirmed hybrid, A132 × (A62 × C1037 L2) D2, was obtained (Supplementary Fig. S1).
As the three-parent hybrid was still POF+, from containing functional PAD1 and FDC1 genes from the A62 × C1037 hybrid, it was also sporulated in an attempt to obtain POF− spore clones. Spore viability was low, and only 4 out of 24 possible spore clones were isolated. These spore clones were screened for the POF phenotype, and three out of four spore clones were POF− (Fig. 5B). Two of the three POF− spore clones also showed the highest apparent β-lyase activity as well (Fig. 5A).
The ability of the three-parent hybrid and derived spore clones to A grow on 15 mM cysteine as a sole nitrogen source and B produce phenolic off-flavour by converting ferulic acid (decrease in absorbance at 320 nm). C The mass lost as CO2 (%) during 80 mL-scale fermentation of 15°Plato wort with parent strain, hybrids, and four spore clones. Strains are coloured based on type: blue, lager; red, ale; orange, spore clone. Different letters above bars indicate significant differences between values as determined by one-way ANOVA and Tukey’s post hoc test (p < 0.05)
Small-scale wort fermentations were also performed to ensure sufficient fermentation performance of the spore clones. The three-parent hybrid fermented the fastest and reached the highest attenuation, followed by the original A62 × C1037 hybrid (Fig. 5C). The spore clones fermented the slowest out of the compared strains, but ‘D2 C4’ was identified as the spore clone with the best fermentation performance. This strain was also POF− and showed good β-lyase activity. The strain fermented as fast as the S. cerevisiae A132 parent strain, but reached a higher attenuation thanks to the ability to use maltotriose.
Whole-genome sequencing of hybrids A62 × C1037 L2, A132 × (A62 × C1037 L2) D2, and A132 × (A62 × C1037 L2) D2 C4 was also performed to examine the genome structure of these hybrids and confirm mutations in the POF-related genes. De novo assemblies of the parent strains S. cerevisiae A132 and S. uvarum C1037 were first generated from long sequencing reads, while a de novo assembly of S. cerevisiae A62 was obtained from Krogerus et al. [43]. The resulting assemblies of 48 and 19 scaffolds for S. cerevisiae A132 and S. uvarum C1037 (including the 16 chromosomes and mitochondrial DNA) and spanned genome sizes of 12.5 and 12.1 Mbp, respectively (assembly statistics and dot plots are available in Supplementary Table S2 and Supplementary Figures S2-3). For analysis of the hybrid strains, a concatenated reference genome of S. cerevisiae A62 and S. uvarum C1037 was used. The A62 × C1037 L2 hybrid produced through rare mating appeared to be tetraploid with nearly two copies of all chromosomes derived from S. cerevisiae and S. uvarum (Fig. 6A). The three-parent hybrid A132 × (A62 × C1037 L2) D2 also appeared to be tetraploid, but rather had approximately three copies of all S. cerevisiae chromosomes and one copy of S. uvarum chromosomes (Fig. 6B). This is expected from spore-to-spore mating of the tetraploid S. cerevisiae A132 and the tetraploid A62 × C1037 L2 hybrid. Finally, the POF− spore clone A132 × (A62 × C1037 L2) D2 C4 appeared diploid, and the strain had lost numerous S. uvarum chromosomes completely, while most S. cerevisiae chromosomes were present in two copies (Fig. 6C). This included S. uvarum chromosome 12, which carries the S. uvarum orthologue of IRC7, and S. uvarum chromosome 13, which carries the S. uvarum orthologues of PAD1 and FDC1. The S. cerevisiae A132 parent strain carries a loss-of-function and frameshift mutation (460C > T, Gln154* and 501dupA, Trp168fs, respectively) in the FDC1 gene, rendering the strain POF−. The same mutations were found in the three-parent hybrid A132 × (A62 × C1037 L2) D2 and its POF− spore clone D2 C4 (Fig. 6D). However, they were heterozygous in D2 (i.e. functional alleles of FDC1 were still present), while homozygous in D2 C4 (i.e. only non-functional present). In both the S. cerevisiae parent strains A62 and A132, the β-lyase coding IRC7 gene carries an inactivating 38-bp deletion. However, the deletion is heterozygous in A62, meaning the full-length IRC7 was present in one copy in all three hybrid strains L2, D2 and D2 C4 (Fig. 6E).
Whole-genome sequencing of three hybrid strains. A–C The estimated chromosome copy numbers of hybrid strains hybrids A A62 × C1037 L2, B A132 × (A62 × C1037 L2) D2, and C A132 × (A62 × C1037 L2) D2 C4. The copy numbers were estimated by normalising the median sequencing coverage in 10 kb windows to the median sequencing coverage across the whole-genome. S. cerevisiae and S. uvarum chromosomes are coloured alternating red–black and blue–black, respectively. D The allele frequencies of nonsense mutations in S. cerevisiae FDC1 detected in the three hybrid strains. E The sequencing coverage along S. cerevisiae IRC7 in the three hybrid strains. The inactivating heterozygous 38-bp deletion occurs around position 288,200 along chromosome VI
10 L-scale fermentations confirm enhanced phenotypes in hybrid and spore clone
To confirm the fermentation phenotypes in larger-scale fermentations, we performed 10 L-scale fermentations in 12°Plato wort with one parent strain (S. cerevisiae A62) along with two hybrids (A62 × C1037 and the POF − ‘D2 C4’ spore clone). Fermentations proceeded as observed previously at smaller scale, with all strains ultimately reaching the same attenuation, but the hybrids reaching it slightly faster (Fig. 7A). The high attenuation (> 85%) indicated maltotriose use by all strains, and this was confirmed by HPLC analysis (data not shown).
Fermentation performance and concentrations of thiols, esters and 4-vinylguaiacol in 10 L-scale fermentations of 12°Plato wort. A Alcohol by volume (%) during fermentations. Concentrations (mg/L) of B 3-methylbutyl acetate, C ethyl hexanoate, D ethyl octanoate, and E 4-vinyl guaiacol in the beers. Concentrations (ng/L) of F 4-mercapto-4-methyl-2-pentanone (4MMP), G 3-mercaptohexanol (3MH), and H 3-mercaptohexylacetate (3MHA) in the beers. Different letters indicate significant differences (p < 0.05) as determined by one-way ANOVA and Tukey’s post hoc test
In regards to aroma formation, significantly less 4-vinyl guaiacol was found in the beer produced with the ‘D2 C4’ spore clone (Fig. 7E). Hence, confirming the POF phenotype that was observed earlier during the POF assay and genome sequencing. In regards to aroma-active esters, the beers made with the hybrid strains contained significantly higher concentrations of 3-methylbutyl acetate, while few differences were observed for the ethyl esters (Fig. 7B–D). The ‘D2 C4’ spore clone was, therefore, not only POF−, but also produced more desirable fruity esters than the other strains here.
Like with the esters, thiols levels were also higher in the beers produced with the hybrid strains (Fig. 7F–H). The beers fermented with the hybrid strains showed significantly higher concentrations of 4MMP, 3MH and 3MHA compared to the parent strain (no difference in 4MMP concentration between A62 and ‘D2 C4’). In the beer fermented with the hybrid strains, concentrations of all three thiols were above or around their flavour thresholds of 1–1.5, 55–60, and 4 ng/L for 4MMP, 3MH, and 3MHA, respectively [15, 49]. This suggested that the thiols had a positive influence on flavour. Thiol levels were lower in the beer fermented with the spore clone, compared to that with the A62 × C1037 hybrid. As β-lyase activity was strongly linked with the S. uvarum parent, it is likely that this decrease in thiol release is due to the loss of S. uvarum IRC7.
Discussion
As a result of growing demand for highly hopped beer with fruity aroma, much attention has been placed on maximising and modifying the aroma obtained from hops. In addition to adjusting hop amount, hop variety and timing of the hop additions, the aroma compounds derived from the hops can also be modified through yeast-mediated enzymatic reactions. One such class of compounds are volatile thiols, which not only play a central role in contributing fruity hop aroma to beer, but can also be released from odourless precursors through β-lyase activity [12,13,14, 50,51,52,53,54,55,56].
Here, we aimed to generate brewing yeast strains with enhanced ability to release volatile thiols during wort fermentation through interspecific hybridization of S. cerevisiae brewing strains with strains of S. uvarum or S. eubayanus. Interspecific hybridization has been used extensively for brewing yeast strain development in the past decade [29,30,31, 57, 58]. It allows for combining traits from diverse parent strains, can be performed without any genetic engineering, and hybrids may in some cases exhibit hybrid vigour, where they outperform the parent strains in certain traits [7]. The approach has been used, for example, to construct interspecific brewing hybrids with both enhanced wort fermentation and a more diverse aroma profile [25, 30]. The hybrids generated in this study were able to release higher concentrations of 4MMP and 3MH than their S. cerevisiae parent, and in some cases also the S. uvarum parent. The result is in line with a study comparing the performance of a range of S. cerevisiae × S. uvarum hybrids in wine fermentations [34]. There, they observed higher release of 4MMP among the S. uvarum and hybrid strains compared to the S. cerevisiae strains. In addition, we also recently observed enhanced thiol release during beer fermentations with selected intraspecific S. cerevisiae hybrids generated between brewing and wild strains of S. cerevisiae [16]. Interspecific hybridization can, therefore, be used to enhance thiol release during beer fermentations as well.
In Saccharomyces yeasts, β-lyase enzymes responsible for releasing 4MMP and 3MH are coded by IRC7 and STR3 genes [14, 55, 56, 59]. IRC7, in particular, has been shown to be key for thiol release, and the gene is well studied in S. cerevisiae [14, 18, 32, 60]. Irc7p activity varies greatly between strains, and a number of inactivating mutations have been identified [14, 18]. These are particularly prevalent among domesticated strains, and include a 38-bp deletion that significantly decreases enzyme activity [18, 19]. Here, we observed the presence of the 38-bp inactivating deletion in both S. cerevisiae A132 (homozygous) and A62 (heterozygous), which explains the lower thiol concentrations in the beers fermented with these strains. It is unclear what causes the higher β-lyase activity of the S. uvarum strains that was observed here, but it could be simply related to a full-length allele of IRC7, or that the S. uvarum orthologue encodes a protein with higher activity. The activity of S. uvarum β-lyase enzymes have, to our knowledge, not been studied or compared to S. cerevisiae orthologues. Another possibility is that the enhanced β-lyase activity of S. uvarum is related to IRC7 regulation and its genomic position [23]. In S. cerevisiae, IRC7 is located in the sub-telomeric region of chromosome VI (around 5 kbp from the right telomere), and this region is downregulated through silencing by the SIR2-encoded histone deacetylase [61, 62]. In our de novo assembly of S. uvarum C1037, IRC7 was located in the sub-telomeric region of chromosome XII (around 18 kbp from the left end of the assembled chromosome). It remains to be explored in future studies how these locations affect IRC7 expression.
Thiol release during beer fermentations is also affected by process conditions. In a recent study with lager yeast, it was revealed that fermentation temperature has a contrasting effect on the release of cysteinylated compared to glutathionylated thiols [63]. Release of thiols from glutathionylated precursors was enhanced at higher fermentations (24 °C), while release from cysteinylated precursors was higher at lower fermentations (12 °C). Lager yeasts, i.e. natural S. cerevisiae × S. eubayanus hybrids, are known for their cold tolerance, and fermentations are usually carried out at lower temperatures than those with S. cerevisiae ale yeast [64]. This cold tolerance is inherited from the S. eubayanus parent [26, 29]. S. uvarum strains typically also exhibit enhanced cold tolerance compared to S. cerevisiae [25, 65, 66]. While not tested here, interspecific hybrids tend to have a broader temperature range for growth and fermentation compared to the parent strains alone [30, 31, 48, 67]. This could be exploited using the constructed hybrids for both low and high temperature wort fermentations, in order to release different profiles of thiols. Using a low-temperature maturation phase (4 °C) after the primary fermentation was also recently revealed to increase the release of thiols into the beer [68], and S. eubayanus, S. uvarum and their hybrids are more likely to be metabolically active at such low temperatures compared to S. cerevisiae ale strains.
In this study, the majority of the newly generated hybrids were created with rare mating [69]. This technique has numerous advantages. First, in comparison to classic spore-to-spore mating, where parent strains undergo sporulation and loss of heterozygosity prior to mating, rare mating tends to form hybrids that have retained the majority of both parental genomes. Furthermore, rare mating of two diploids form an allotetraploid hybrid, such as A62 × C1037 L2. These allotetraploid hybrids, in contrast to allodiploid hybrids formed by the mating of two haploid spores, often perform better phenotypically [43, 48] and also tend to be fertile and can undergo sporulation [43, 70, 71]. Here, we exploited this fertility by first creating the three-parent hybrid D2, and then removing its heterozygous POF trait in spore clone D2 C4. While not investigated here in much detail, sporulation of de novo hybrids has also been used to generate phenotypic diversity [43]. The spore clone D2 C4 had, for example, lost numerous S. uvarum-derived chromosomes during meiosis, and this is likely to cause phenotypic diversity among ascus siblings. As meiotic chromosome segregation is random, and chromosome loss can occur spontaneously in hybrids [72], a larger set of hybrids and spore clones could be screened if more specific phenotypic properties are desired. Experimental evolution experiments have also shown that exposure to different stresses can lead to partial loss of either parental sub-genome [73,74,75].
To conclude, our study reveals that interspecific hybridization can be used to enhance the β-lyase activity and thiol releasing ability of brewing yeast strains. We observed higher β-lyase activity in the non-cerevisiae Saccharomyces strains compared to S. cerevisiae brewing strains, and hybrids between them were shown to release high amounts of volatile thiols during fermentation. While the study here only focussed on interspecific hybrids with S. uvarum and S. eubayanus, it is possible that other species in the Saccharomyces genus also exhibit higher β-lyase activity compared to S. cerevisiae brewing yeast strains. This could be explored in future studies. As has been seen in previous studies, the constructed hybrid strains here also showed improved fermentation and formation of yeast-derived aroma compounds. Unwanted traits, such as phenolic off-flavour production, could be further removed by exploiting the fertility of the allotetraploid hybrids. Increased thiol release has many advantages for the fermentation of modern hoppy beer. It not only allows for the production of beer with boosted hop aroma, but decreases the environmental impact of the brewing process through lower hop use.
Data availability
The ONT reads generated in this study have been submitted to NCBI-SRA under BioProject number PRJNA820581 in the NCBI BioProject database (https://www.ncbi.nlm.nih.gov/bioproject/).
References
Gonzalez Viejo C, Fuentes S (2020) Beer aroma and quality traits assessment using artificial intelligence. Fermentation 6:56. https://doi.org/10.3390/fermentation6020056
Aquilani B, Laureti T, Poponi S, Secondi L (2015) Beer choice and consumption determinants when craft beers are tasted: an exploratory study of consumer preferences. Food Qual Prefer 41:214–224. https://doi.org/10.1016/j.foodqual.2014.12.005
Carbone A, Quici L (2020) Craft beer mon amour: an exploration of Italian craft consumers. Br Food J 122:2671–2687. https://doi.org/10.1108/BFJ-07-2019-0476
Brewers Association B (2021) Brewers Association 2020 Hop & Brewing Industry Update
Lafontaine SR, Shellhammer TH (2019) How hoppy beer production has redefined hop quality and a discussion of agricultural and processing strategies to promote it. Tech Q. https://doi.org/10.1094/tq-56-1-0221-01
Cubillos FA, Gibson B, Grijalva-Vallejos N et al (2019) Bioprospecting for brewers: exploiting natural diversity for naturally diverse beers. Yeast 36:383–398. https://doi.org/10.1002/yea.3380
Krogerus K, Magalhães F, Vidgren V, Gibson B (2017) Novel brewing yeast hybrids: creation and application. Appl Microbiol Biotechnol 101:65–78. https://doi.org/10.1007/s00253-016-8007-5
Holt S, Mukherjee V, Lievens B et al (2018) Bioflavoring by non-conventional yeasts in sequential beer fermentations. Food Microbiol 72:55–66. https://doi.org/10.1016/j.fm.2017.11.008
Takoi K, Koie K, Itoga Y et al (2010) Biotransformation of hop-derived monoterpene alcohols by lager yeast and their contribution to the flavor of hopped beer. J Agric Food Chem 58:5050–5058. https://doi.org/10.1021/jf1000524
Rettberg N, Biendl M, Garbe L-A (2018) Hop aroma and hoppy beer flavor: chemical backgrounds and analytical tools—a review. J Am Soc Brew Chem 76:1–20. https://doi.org/10.1080/03610470.2017.1402574
Svedlund N, Evering S, Gibson B, Krogerus K (2022) Fruits of their labour: biotransformation reactions of yeasts during brewery fermentation. Appl Microbiol Biotechnol 106(13–16):4929–4944
Roland A, Viel C, Reillon F et al (2016) First identification and quantification of glutathionylated and cysteinylated precursors of 3-mercaptohexan-1-ol and 4-methyl-4-mercaptopentan-2-one in hops (Humulus lupulus). Flavour Fragr J 31:455–463. https://doi.org/10.1002/ffj.3337
Gros J, Peeters F, Collin S (2012) Occurrence of odorant polyfunctional thiols in beers hopped with different cultivars. First evidence of an S -cysteine conjugate in hop (Humulus lupulus L.). J Agric Food Chem 60:7805–7816. https://doi.org/10.1021/jf301478m
Roncoroni M, Santiago M, Hooks DO et al (2011) The yeast IRC7 gene encodes a β-lyase responsible for production of the varietal thiol 4-mercapto-4-methylpentan-2-one in wine. Food Microbiol 28:926–935. https://doi.org/10.1016/j.fm.2011.01.002
Capone DL, Barker A, Williamson PO, Francis IL (2018) The role of potent thiols in Chardonnay wine aroma. Aust J Grape Wine Res 24:38–50. https://doi.org/10.1111/ajgw.12294
Krogerus K, Fletcher E, Rettberg N et al (2021) Efficient breeding of industrial brewing yeast strains using CRISPR/Cas9-aided mating-type switching. Appl Microbiol Biotechnol. https://doi.org/10.1007/s00253-021-11626-y
Curtin C, Vega E, Cordente T, Fortmann K (2020) Mutations in carbon-sulfur ß-lyase encoding gene IRC7 affect the polyfunctional thiol-releasing capability of brewers yeast. In: World Brewing Congress 2020
Cordente AG, Borneman AR, Bartel C et al (2019) Inactivating mutations in Irc7p are common in wine yeasts, attenuating carbon-sulfur β-lyase activity and volatile sulfur compound production. Appl Environ Microbiol. https://doi.org/10.1128/AEM.02684-18
Ruiz J, Celis M, Martín-Santamaría M et al (2021) Global distribution of <scp> IRC7 </scp> alleles in <scp> Saccharomyces cerevisiae </scp> populations: a genomic and phenotypic survey within the wine clade. Environ Microbiol 23:3182–3195. https://doi.org/10.1111/1462-2920.15540
Knight SJ, Klaere S, Morrison-Whittle P, Goddard MR (2018) Fungal diversity during fermentation correlates with thiol concentration in wine. Aust J Grape Wine Res 24:105–112. https://doi.org/10.1111/ajgw.12304
da Silva T, Albertin W, Dillmann C et al (2015) Hybridization within saccharomyces genus results in homoeostasis and phenotypic novelty in winemaking conditions. PLoS ONE 10:e0123834. https://doi.org/10.1371/journal.pone.0123834
Masneuf-Pomarède I, Murat M-L, Naumov GI et al (2002) Hybrids <em>Saccharomyces cerevisiae</em> X <em>Saccharomyces bayanus</em> var. <em>uvarum</em> having a high liberating ability of some sulfur varietal aromas of <em>Vitis vinifera</em> Sauvignon blanc wines. OENO One 36:205
Holt S, Miks MH, de Carvalho BT et al (2019) The molecular biology of fruity and floral aromas in beer and other alcoholic beverages. FEMS Microbiol Rev 43:193–222. https://doi.org/10.1093/femsre/fuy041
Libkind D, Hittinger CT, Valério E et al (2011) Microbe domestication and the identification of the wild genetic stock of lager-brewing yeast. Proc Natl Acad Sci U S A 108:14539–14544. https://doi.org/10.1073/pnas.1105430108
Nikulin J, Krogerus K, Gibson B (2018) Alternative Saccharomyces interspecies hybrid combinations and their potential for low-temperature wort fermentation. Yeast 35:113–127. https://doi.org/10.1002/yea.3246
Gibson BR, Storgårds E, Krogerus K, Vidgren V (2013) Comparative physiology and fermentation performance of Saaz and Frohberg lager yeast strains and the parental species Saccharomyces eubayanus. Yeast 30:255–266. https://doi.org/10.1002/yea.2960
Burini JA, Eizaguirre JI, Loviso C, Libkind D (2022) Selection of Saccharomyces eubayanus strains from Patagonia (Argentina) with brewing potential and performance in the craft beer industry. Eur Food Res Technol 248:519–531. https://doi.org/10.1007/s00217-021-03897-6
Mardones W, Villarroel CA, Krogerus K et al (2020) Molecular profiling of beer wort fermentation diversity across natural Saccharomyces eubayanus isolates. Microb Biotechnol 13:1012–1025. https://doi.org/10.1111/1751-7915.13545
Krogerus K, Magalhães F, Vidgren V, Gibson B (2015) New lager yeast strains generated by interspecific hybridization. J Ind Microbiol Biotechnol 42:769–778. https://doi.org/10.1007/s10295-015-1597-6
Mertens S, Steensels J, Saels V et al (2015) A large set of newly created interspecific Saccharomyces hybrids increases aromatic diversity in lager beers. Appl Environ Microbiol 81:8202–8214. https://doi.org/10.1128/AEM.02464-15
Krogerus K, Seppänen-Laakso T, Castillo S, Gibson B (2017) Inheritance of brewing-relevant phenotypes in constructed Saccharomyces cerevisiae x Saccharomyces eubayanus hybrids. Microb Cell Fact 16:66. https://doi.org/10.1186/s12934-017-0679-8
Dufour M, Zimmer A, Thibon C, Marullo P (2013) Enhancement of volatile thiol release of Saccharomyces cerevisiae strains using molecular breeding. Appl Microbiol Biotechnol 97:5893–5905. https://doi.org/10.1007/s00253-013-4739-7
Masneuf I, Murat ML, Naumov GI et al (2002) Hybrids saccharomyces cerevisiae x Saccharomyces bayanus var. uvarum having a high liberating ability of some sulfur varietal aromas of vitis vinifera sauvignon blanc wines. J Int des Sci la Vigne du Vin. https://doi.org/10.20870/oeno-one.2002.36.4.965
Da Silva T, Albertin W, Dillmann C et al (2015) Hybridization within Saccharomyces genus results in homoeostasis and phenotypic novelty in winemaking conditions. PLoS ONE. https://doi.org/10.1371/journal.pone.0123834
Boeke JD, Trueheart J, Natsoulis G, Fink GR (1987) [10] 5-Fluoroorotic acid as a selective agent in yeast molecular genetics. Methods Enzymol 154:164–175. https://doi.org/10.1016/0076-6879(87)54076-9
Zaret KS, Sherman F (1985) ??-Aminoadipate as a primary nitrogen source for Saccharomyces cerevisiae mutants. J Bacteriol 162:579–583
Pham T, Wimalasena T, Box WG et al (2011) Evaluation of ITS PCR and RFLP for differentiation and identification of brewing yeast and brewery ‘wild’ yeast contaminants. J Inst Brew 117:556–568. https://doi.org/10.1002/j.2050-0416.2011.tb00504.x
Ellman GL (1958) A colorimetric method for determining low concentrations of mercaptans. Arch Biochem Biophys 74:443–450. https://doi.org/10.1016/0003-9861(58)90014-6
Mertens S, Steensels J, Gallone B et al (2017) Rapid screening method for phenolic off-flavor (POF) production in yeast. J Am Soc Brew Chem 75:318–323. https://doi.org/10.1094/ASBCJ-2017-4142-01
Rodriguez-Bencomo JJ, Muñoz-González C, Martín-Álvarez PJ et al (2012) Optimization of a HS-SPME-GC-MS procedure for beer volatile profiling using response surface methodology: application to follow aroma stability of beers under different storage conditions. Food Anal Methods 5:1386–1397. https://doi.org/10.1007/s12161-012-9390-x
Denis E, Sanchez S, Mairey B et al (2018) Extracting high molecular weight genomic DNA from Saccharomyces cerevisiae. Protoc Exch. https://doi.org/10.1038/protex.2018.076
Kolmogorov M, Yuan J, Lin Y, Pevzner PA (2019) Assembly of long, error-prone reads using repeat graphs. Nat Biotechnol 37:540–546. https://doi.org/10.1038/s41587-019-0072-8
Krogerus K, Magalhães F, Castillo S et al (2021) Lager yeast design through meiotic segregation of a Saccharomyces cerevisiae × Saccharomyces eubayanus hybrid. Front Fungal Biol. https://doi.org/10.3389/ffunb.2021.733655
Li H (2018) Minimap2: pairwise alignment for nucleotide sequences. Bioinformatics 34:3094–3100. https://doi.org/10.1093/bioinformatics/bty191
Pedersen BS, Quinlan AR (2018) Mosdepth: quick coverage calculation for genomes and exomes. Bioinformatics 34:867–868. https://doi.org/10.1093/bioinformatics/btx699
Luo R, Wong C-L, Wong Y-S et al (2020) Exploring the limit of using a deep neural network on pileup data for germline variant calling. Nat Mach Intell 2:220–227. https://doi.org/10.1038/s42256-020-0167-4
Santiago M, Gardner RC (2015) The IRC7 gene encodes cysteine desulphydrase activity and confers on yeast the ability to grow on cysteine as a nitrogen source. Yeast. https://doi.org/10.1002/yea.3076
Krogerus K, Arvas M, De Chiara M et al (2016) Ploidy influences the functional attributes of de novo lager yeast hybrids. Appl Microbiol Biotechnol 100:7203–7222. https://doi.org/10.1007/s00253-016-7588-3
Kishimoto T, Wanikawa A, Kono K, Shibata K (2006) Comparison of the odor-active compounds in unhopped beer and beers hopped with different hop varieties. J Agric Food Chem 54:8855–8861. https://doi.org/10.1021/jf061342c
Dennenlöhr J, Thörner S, Rettberg N (2020) Analysis of hop-derived thiols in beer using on-fiber derivatization in combination with HS-SPME and GC-MS/MS. J Agric Food Chem 68:15036–15047. https://doi.org/10.1021/acs.jafc.0c06305
Cibaka M-LK, Ferreira CS, Decourrière L et al (2017) Dry hopping with the dual-purpose varieties amarillo, citra, hallertau blanc, mosaic, and sorachi ace: minor contribution of hop terpenol glucosides to beer flavors. J Am Soc Brew Chem 75:122–129. https://doi.org/10.1094/ASBCJ-2017-2257-01
Bonnaffoux H, Roland A, Schneider R, Cavelier F (2021) Spotlight on release mechanisms of volatile thiols in beverages. Food Chem 339:127628. https://doi.org/10.1016/j.foodchem.2020.127628
Michel M, Haslbeck K, Ampenberger F et al (2019) Screening of brewing yeast β-lyase activity and release of hop volatile thiols from precursors during fermentation. BrewingScience 72:179–186. https://doi.org/10.23763/BrSc19-26michel
Chenot C, Robiette R, Collin S (2019) First evidence of the cysteine and glutathione conjugates of 3-sulfanylpentan-1-ol in Hop (Humulus lupulus L.). J Agric Food Chem 67:4002–4010. https://doi.org/10.1021/acs.jafc.9b00225
Howell KS, Klein M, Swiegers JH et al (2005) Genetic determinants of volatile-thiol release by saccharomyces cerevisiae during wine fermentation. Appl Environ Microbiol 71:5420–5426. https://doi.org/10.1128/AEM.71.9.5420-5426.2005
Holt S, Cordente AG, Williams SJ et al (2011) Engineering Saccharomyces cerevisiae to release 3-mercaptohexan-1-ol during fermentation through overexpression of an S. cerevisiae gene, STR3, for improvement of wine aroma. Appl Environ Microbiol. https://doi.org/10.1128/AEM.03009-10
Ota T, Kanai K, Nishimura H et al (2021) Generation of new hybrids by crossbreeding between bottom-fermenting yeast strains. J Biosci Bioeng 131:61–67. https://doi.org/10.1016/j.jbiosc.2020.08.009
Catallo M, Iattici F, Randazzo CL et al (2021) Hybridization of Saccharomyces cerevisiae Sourdough strains with cryotolerant Saccharomyces bayanus NBRC1948 as a strategy to increase diversity of strains available for lager beer fermentation. Microorganisms 9:514. https://doi.org/10.3390/microorganisms9030514
Thibon C, Marullo P, Claisse O et al (2008) Nitrogen catabolic repression controls the release of volatile thiols by Saccharomyces cerevisiae during wine fermentation. FEMS Yeast Res 8:1076–1086. https://doi.org/10.1111/j.1567-1364.2008.00381.x
Santiago M, Gardner RC (2015) Yeast genes required for conversion of grape precursors to varietal thiols in wine. FEMS Yeast Res 15:fov034. https://doi.org/10.1093/femsyr/fov034
Ehrentraut S, Weber JM, Dybowski JN et al (2010) Rpd3-dependent boundary formation at telomeres by removal of Sir2 substrate. Proc Natl Acad Sci 107:5522–5527. https://doi.org/10.1073/pnas.0909169107
Samel A, Rudner A, Ehrenhofer-Murray AE (2017) Variants of the Sir4 coiled-coil domain improve binding to sir3 for heterochromatin formation in Saccharomyces cerevisiae. G3 Genes Genomes Genetics 7:1117–1126. https://doi.org/10.1534/g3.116.037739
Chenot C, Donck W, Janssens P, Collin S (2022) Malt and hop as sources of thiol S -conjugates: thiol-releasing property of lager yeast during fermentation. J Agric Food Chem 70:3272–3279. https://doi.org/10.1021/acs.jafc.1c07272
Gibson B, Liti G (2015) Saccharomyces pastorianus: genomic insights inspiring innovation for industry. Yeast 32:17–27. https://doi.org/10.1002/yea.3033
Gallone B, Steensels J, Mertens S et al (2019) Interspecific hybridization facilitates niche adaptation in beer yeast. Nat Ecol Evol 3:1562–1575. https://doi.org/10.1038/s41559-019-0997-9
Paget CM, Schwartz JM, Delneri D (2014) Environmental systems biology of cold-tolerant phenotype in Saccharomyces species adapted to grow at different temperatures. Mol Ecol 23:5241–5257. https://doi.org/10.1111/mec.12930
Baker EP, Peris D, Moriarty RV et al (2019) Mitochondrial DNA and temperature tolerance in lager yeasts. Sci Adv 5:eaav1869. https://doi.org/10.1126/sciadv.aav1869
Chenot C, Thibault de Chanvalon E, Janssens P, Collin S (2021) Modulation of the sulfanylalkyl acetate/alcohol ratio and free thiol release from cysteinylated and/or glutathionylated sulfanylalkyl alcohols in beer under different fermentation conditions. J Agric Food Chem 69:6005–6012. https://doi.org/10.1021/acs.jafc.1c01610
Gunge N, Nakatomi Y (1972) Genetic mechanisms of rare matings of the yeast Saccharomyces cerevisiae heterozygous for mating type. Genetics 70:41–58
Greig D, Borts RH, Louis EJ, Travisano M (2002) Epistasis and hybrid sterility in Saccharomyces. Proc Biol Sci 269:1167–1171. https://doi.org/10.1098/rspb.2002.1989
Naseeb S, Visinoni F, Hu Y et al (2021) Restoring fertility in yeast hybrids: breeding and quantitative genetics of beneficial traits. Proc Natl Acad Sci. https://doi.org/10.1073/pnas.2101242118
Kumaran R, Yang SY, Leu JY (2013) Characterization of chromosome stability in diploid polyploid and hybrid yeast cells. PLoS ONE. https://doi.org/10.1371/journal.pone.0068094
Piotrowski JS, Nagarajan S, Kroll E et al (2012) Different selective pressures lead to different genomic outcomes as newly-formed hybrid yeasts evolve. BMC Evol Biol 12:46. https://doi.org/10.1186/1471-2148-12-46
Dunn B, Paulish T, Stanbery A et al (2013) Recurrent rearrangement during adaptive evolution in an interspecific yeast hybrid suggests a model for rapid introgression. PLoS Genet 9:e1003366. https://doi.org/10.1371/journal.pgen.1003366
Krogerus K, Holmström S, Gibson B (2018) Enhanced wort fermentation with de novo lager hybrids adapted to high-ethanol environments. Appl Environ Microbiol 84:e02302-e2317. https://doi.org/10.1128/AEM.02302-17
Krogerus K, Eerikäinen R, Aisala H, Gibson B (2022) Repurposing brewery contaminant yeast as production strains for low-alcohol beer fermentation. Yeast 39:156–169. https://doi.org/10.1002/yea.3674
Acknowledgements
We thank Aila Siltala, Niklas Fred, Eero Mattila and Ronja Eerikäinen for technical assistance. Benjamin Palmer and Sarah Thörner are acknowledged for thiol analysis.
Funding
Open Access funding provided by Technical Research Centre of Finland (VTT). The study was funded by PBL Brewing Laboratory and Business Finland.
Author information
Authors and Affiliations
Contributions
KK: conceived the study, designed experiments, performed experiments, analysed all data, and wrote the manuscript. NR: supervised thiol analysis and edited the manuscript. BG: conceived the study, designed experiments, and edited the manuscript. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Kristoffer Krogerus and Brian Gibson were employed by VTT Technical Research Centre of Finland Ltd. Nils Rettberg was employed by VLB Berlin. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contribution for the Special Issue: The chemistry behind malt and beer production – from raw material to product quality.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Krogerus, K., Rettberg, N. & Gibson, B. Increased volatile thiol release during beer fermentation using constructed interspecies yeast hybrids. Eur Food Res Technol 249, 55–69 (2023). https://doi.org/10.1007/s00217-022-04132-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-022-04132-6