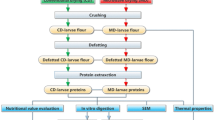
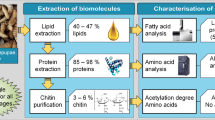
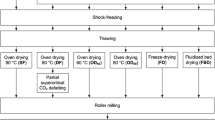
Abstract
The emergence of black soldier fly larvae (BSFL) as one among the vital tools for generating circular economy has enabled its use in several applications such as biorefining, valorization of waste management, treatment of industrial by-products, and bioconversion of agro-industrial residues. The ability of BSFL to assimilate chemical constituents of interest from the feed substrate and easily recover them from the insect matrix gives it a bio-reactor like characteristic. The primary unit operation after insect harvesting is the devitalization technique employed, and further processing of BSFL depends on the end application of the insect product. Conventional drying, microwave drying, scalding, blanching, microwave-assisted subcritical condition, and freezing as devitalization techniques to euthanize BSFL was executed. The impact of the devitalization techniques on the BSFL oil, protein, antioxidant, and chitin fractions was evaluated. The crude lipid yield, fatty acid profile and lipid class composition were investigated. The fatty acid profile was uniform for BSFL fractions devitalized by different techniques, and triacylglycerides were the primary lipid class in the oil fraction. The protein solubility of the defatted BSFL flour in different pH was determined. The protein quality parameters such as protein dispersibility index, urease activity index, and molecular weight distribution of the soluble protein fractions were analyzed. The frozen BSFL fraction displayed the highest protein solubility in all pH range considered, protein dispersibility index (PDI) and protein solubility (PS) was 52.86 ± 2.99% and 83.94 ± 2.96%, respectively. The aminogram of dry defatted BSFL flour, BSFL concentrate and isolate were recorded and the nitrogen-to-protein conversion (Kp) value was determined for these fractions. The antioxidant capacity of water-soluble bioactive compounds of BSFL was evaluated. Radical scavenging capacity and total polyphenol content of the BSFL fraction devitalized by microwave-assisted subcritical treatment and freezing displayed higher values when compared to other BSFL fractions. The chitin fractions were characterized by determining the degree of acetylation. Devitalization techniques strongly influence and impact the major BSFL constituents except for chitin and selection of the method should depend on the end application of the insect product. The microwave-assisted subcritical treatment proved to be an efficient devitalization technique. Whereas, freezing being a non-thermal treatment has a few limitations.
Graphic abstract









Similar content being viewed by others
References
Cappelli A, Cini E, Lorini C, Oliva N, Bonaccorsi G (2020) Insects as food: A review on risks assessments of Tenebrionidae and Gryllidae in relation to a first machines and plants development. Food Control 108:106877
Delgado C, Rosegrant M, Steinfeld H, Ehui S (2020) Livestock to 2020 : the next food revolution, pp 27–29
Kyriakopoulou K, Dekkers B, Van Der Goot AJ (2019) Plant-based meat analogues, sustainable meat production and processing. Elsevier Inc. 10.1016/B978-0-12-814874-7.00006-7
European Commission (2017) Commission Regulation (EU) No. 2017/893 of 24 May 2017 amending Annexes I and IV to Regulation (EC) No. 999/2001 of the European Parliament and of the Council and Annexes X, XIV and XV to Commission Regulation (EU) No. 142/2011 as regards the provisions on processed animal protein. Official Journal L 138/92, 1–25
European Commission (2009) Commission Regulation (EU) No. 1069/2009 of the European Parliament and of the Council of 21 October 2009 laying down health rules as regards animal by-products and derived products not intended for human consumption and repealing Regulation (EC) No. 1774/2002 (Animal by-products Regulation). Official Journal L 300/1, 1–33
European Commission (2001) Commission Regulation (EU) No. 999/2001 of the European Parliament and of the Council of 22 May 2001 laying down rules for the prevention, control and eradication of certain transmissible spongiform encephalopathies. Official Journal L 147, 1–40
Pinotti L, Giromini C, Ottoboni M, Tretola M, Marchis D (2019) Review: insects and former foodstuffs for upgrading food waste biomasses/streams to feed ingredients for farm animals, pp 1365–1375. https://doi.org/10.1017/S1751731118003622
Barrows FT, Bellis D, Krogdahl Å, Silverstein JT, Eliot M, Sealey WM, Rust MB, Iii DMG (2008) Reviews in fisheries science report of the plant products in aquafeed strategic planning workshop: an integrated interdisciplinary research roadmap for increasing utilization of plant feedstuffs in diets for carnivorous fish. Rev Fish Sci. https://doi.org/10.1080/10641260802046734
Sánchez-muros M, Barroso FG, Manzano-agugliaro F (2014) Insect meal as renewable source of food for animal feeding: a review. J Clean Prod 65:16–27. https://doi.org/10.1016/j.jclepro.2013.11.068
Caimi C, Renna M, Lussiana C, Bonaldo A, Gariglio M, Meneguz M, Dabbou S, Schiavone A, Gai F, Concetta A, Prearo M, Gasco L (2020) First insights on Black Soldier Fly (Hermetia illucens L.) larvae meal dietary administration in Siberian sturgeon (Acipenser baerii Brandt) juveniles. Aquaculture 515:734539. https://doi.org/10.1016/j.aquaculture.2019.734539
Kroeckel S, Harjes AE, Roth I, Katz H, Wuertz S, Susenbeth A, Schulz C (2012) When a turbot catches a fly: evaluation of a pre-pupae meal of the Black Soldier Fly (Hermetia illucens) as fish meal substitute—growth performance and chitin degradation in juvenile turbot (Psetta maxima). Aquaculture 364–365:345–352. https://doi.org/10.1016/j.aquaculture.2012.08.041
Belghit I, Liland NS, Gjesdal P, Biancarosa I, Menchetti E, Li Y, Waagbø R, Krogdahl Å, Lock E (2019) Black soldier fly larvae meal can replace fish meal in diets of sea-water phase Atlantic salmon (Salmo salar). Aquaculture 503:609–619. https://doi.org/10.1016/j.aquaculture.2018.12.032
Cappelli A, Oliva N, Bonaccorsi G, Lorini C, Cini E (2020) Assessment of the rheological properties and bread characteristics obtained by innovative protein sources (Cicer arietinum, Acheta domesticus, Tenebrio molitor): novel food or potential improvers for wheat flour? Lwt 118:108867. https://doi.org/10.1016/j.lwt.2019.108867
Oonincx DGAB, Itterbeeck JV, Heetkamp MJW, Brand HV, Den L, Van JJA, Huis AV (2010) An exploration on greenhouse gas and ammonia production by insect species suitable for animal or human consumption. PLoS ONE 5:1–7. https://doi.org/10.1371/journal.pone.0014445
Huis AV, Oonicx GABD (2017) The environmental sustainability of insects as food and feed. A review. Agron Sustain Dev. https://doi.org/10.1007/s13593-017-0452-8
Smetana S, Schmitt E, Mathys A (2019) Resources, conservation & recycling sustainable use of Hermetia illucens insect biomass for feed and food: attributional and consequential life cycle assessment. Resour Conserv Recycl 144:285–296. https://doi.org/10.1016/j.resconrec.2019.01.042
Caligiani A, Marseglia A, Sorci A, Bonzanini F, Lolli V, Maistrello L, Sforza S (2018) Influence of the killing method of the black soldier fly on its lipid composition. Food Res Int. https://doi.org/10.1016/j.foodres.2018.08.033
Leni G, Caligiani A, Sforza S (2019) Killing method affects the browning and the quality of the protein fraction of Black Soldier Fly (Hermetia illucens) prepupae: a metabolomics and proteomic insight. Food Res Int 115:116–125. https://doi.org/10.1016/j.foodres.2018.08.021
Larouche J, Deschamps M-H, Saucier L, Lebeuf Y, Doyen A, Vandenberg GW (2019) Effects of killing methods on lipid oxidation, colour and microbial load of Black Soldier Fly (Hermetia illucens) larvae. Animals 9:182. https://doi.org/10.3390/ani9040182
AOAC (1990) Official method of analysis of the Association of Official Analytical Chemists. AOAC, Arlington
Caligiani A, Marseglia A, Leni G, Baldassarre S, Maistrello L (2018) Composition of black soldier fly prepupae and systematic approaches for extraction and fractionation of proteins, lipids and chitin. Food Res Int 105:812–820. https://doi.org/10.1016/j.foodres.2017.12.012
Van Eys JE (2012) Manual of quality analyses for soybean products in the feed industry, 2nd edn. U.S. Soybean Export Council
Janssen RH, Lakemond CMM, Fogliano V, Vincken J-P, van den Broek LAM (2017) Nitrogen-to-protein conversion factors for three edible insects: Tenebrio molitor, Alphitobius diaperinus, and Hermetia illucens. J Agric Food Chem 65:2275–2278. https://doi.org/10.1021/acs.jafc.7b00471
Purschke B, Meinlschmidt P, Horn C, Rieder O, Jäger H (2018) Improvement of techno-functional properties of edible insect protein from migratory locust by enzymatic hydrolysis. Eur Food Res Technol 244:999–1013. https://doi.org/10.1007/s00217-017-3017-9
Mattia CD, Battista N, Sacchetti G, Serafini M (2019) Antioxidant activities in vitro of water and liposoluble extracts obtained by different species of edible insects and invertebrates. Front Nutr 6:1–7. https://doi.org/10.3389/fnut.2019.00106
Ravi HK, Breil C, Vian MA, Chemat F, Venskutonis PR (2018) Biorefining of bilberry (Vaccinium myrtillus L.) pomace using microwave hydrodiffusion and gravity, ultrasound-assisted, and bead-milling extraction. ACS Sustain Chem Eng 6:4185–4193. https://doi.org/10.1021/acssuschemeng.7b04592
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 26:1231–1237
Benzie IF, Strain J (1996) The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem 239:70–76
Brugnerotto J, Lizardi J, Goycoolea FM, Argüelles-Monal W, Desbrières J, Rinaudo M (2001) An infrared investigation in relation with chitin and chitosan characterization. Polymer 42:3569–3580. https://doi.org/10.1016/S0032-3861(00)00713-8
Smets R, Verbinnen B, Voorde I, Van De Aerts G, Claes J, Van Der Borght M (2020) Sequential extraction and characterisation of lipids, proteins, and chitin from Black Soldier Fly (Hermetia illucens) larvae, prepupae, and pupae. Waste Biomass Valoriz. https://doi.org/10.1007/s12649-019-00924-2
Aziz A, Zheng L, Cai M, Xiao X, Hu S, Mathys A, Gold M, Yu Z, Zhang J (2019) Influence of Lactobacillus buchneri on soybean curd residue co-conversion by black soldier fly larvae (Hermetia illucens) for food and feedstock production. Waste Manage 86:114–122. https://doi.org/10.1016/j.wasman.2019.01.022
Jonathan AA, Funmilola AS (2014) Nutritional and anti-nutritional composition of Bridelia ferruginea Benth (Euphorbiaceae) stem bark sample. Int J Sci Res Knowl 2:92–104
Xu B, Chang SKC (2009) Phytochemical profiles and health-promoting effects of cool-season food legumes as influenced by thermal processing. J Agric Food Chem 57:10718–107131
Ravi HK, Vian MA, Tao Y, Degrou A (2019) Alternative solvents for lipid extraction and their effect on protein quality in black soldier fly (Hermetia illucens) larvae. J Clean Prod. https://doi.org/10.1016/j.jclepro.2019.117861
Liland NS, Biancarosa I, Araujo P, Biemans D, Bruckner CG, Waagbø R, Torstensen BE, Lock EJ (2017) Modulation of nutrient composition of black soldier fly (Hermetia illucens) larvae by feeding seaweed-enriched media. PLoS ONE 12:1–23. https://doi.org/10.1371/journal.pone.0183188
Ravi HK, Degrou A, Costil J, Trespeuch C (2020) Larvae mediated valorization of industrial agriculture and food wastes: biorefinery concept through bioconversion, processes, procedures, and products. Processes. https://doi.org/10.3390/pr8070857
Wong C, Rosli S, Uemura Y, Ho YC (2019) Potential Protein and Biodiesel Sources from Black Soldier Fly Larvae: insights of larval harvesting instar and fermented feeding medium
Feng W, Xiong H, Wang W, Duan X, Yang T, Wu C, Yang F, Wang T, Wang C (2020) A facile and mild one-pot process for direct extraction of lipids from wet energy insects of black soldier fly larvae. Renew Energy 147:584–593. https://doi.org/10.1016/j.renene.2019.08.137
Zheng L, Li Q, Zhang J, Yu Z (2012) Double the biodiesel yield: Rearing black soldier fly larvae, Hermetia illucens, on solid residual fraction of restaurant waste after grease extraction for biodiesel production. Renew Energy 41:75–79. https://doi.org/10.1016/j.renene.2011.10.004
Su C, Nguyen HC, Bui TL, Huang D (2019) Enzyme-assisted extraction of insect fat for biodiesel production. J Clean Prod 223:436–444. https://doi.org/10.1016/j.jclepro.2019.03.150
Surendra KC, Olivier R, Tomberlin JK, Jha R, Kumar S (2016) Bioconversion of organic wastes into biodiesel and animal feed via insect farming. Renew Energy 98:197–202. https://doi.org/10.1016/j.renene.2016.03.022
Wang C, Qian L, Wang W, Wang T, Deng Z, Yang F, Xiong J, Feng W (2017) Exploring the potential of lipids from black soldier fly: new paradigm for biodiesel production (I). Renew Energy 111:749–756. https://doi.org/10.1016/j.renene.2017.04.063
Chinh H, Liang S, Li S, Su C (2018) Journal of the Taiwan Institute of Chemical Engineers Direct transesterification of black soldier fly larvae (Hermetia illucens) for biodiesel production. J Taiwan Inst Chem Eng 85:165–169. https://doi.org/10.1016/j.jtice.2018.01.035
Sangduan C, Sai S (2018) Skincare products containing Hermetia illucens extract. US 2018/0256483 A1
Matthäus B, Piofczyk T, Katz H, Pudel F (2019) Renewable resources from insects: exploitation, properties, and refining of fat obtained by cold-pressing from Hermetia illucens (Black Soldier Fly) larvae. Eur J Lipid Sci Technol. https://doi.org/10.1002/ejlt.201800376
Roth FX, Kirchgessner M (1998) Organic acids as feed additives for young pigs: nutritional and gastrointestinal effects. J Anim Feed Sci 7:25–33
Soetemans L, Uyttebroek M, Hondt ED, Bastiaens L (2019) Use of organic acids to improve fractionation of the black soldier fly larvae juice into lipid- and protein-enriched fractions. Eur Food Res Technol. https://doi.org/10.1007/s00217-019-03328-7
Zayas JF (1997) Solubility of proteins. In: Functionality of proteins in food. Springer, Berlin. https://doi.org/10.1007/978-3-642-59116-7_2
Bußler S, Rumpold BA, Jander E, Rawel HM (2016) Recovery and techno-functionality of flours and proteins from two edible insect species: Mealworm (Tenebrio molitor) and black soldier fly (Hermetia illucens) larvae. Heliyon. https://doi.org/10.1016/j.heliyon.2016.e00218
Janssen RH, Canelli G, Sanders MG, Bakx EJ, Lakemond CMM, Fogliano V, Vincken J (2019) Iron-polyphenol complexes cause blackening upon grinding Hermetia illucens (black soldier fly) larvae. Sci Rep. https://doi.org/10.1038/s41598-019-38923-x
Yi L, Boekel MAJS, Lakemond CMM (2017) Extracting Tenebrio molitor protein while preventing browning: effect of pH and NaCl on protein yield. J Insects Food Feed 3:21–31. https://doi.org/10.3920/JIFF2016.0015
Andersen SO (2010) Insect cuticular sclerotization: a review. Insect Biochem Mol Biol 40:166–178. https://doi.org/10.1016/j.ibmb.2009.10.007
Sugumaran M (2002) Comparative biochemistry of eumelanogenesis and the protective roles of phenoloxidase and melanin in insects. Pigment Cell Res 15:2–9
Salazar-villanea S, Bruininx EMAM, Gruppen H, Hendriks WH, Carré P, Quinsac A, Van Der Poel AFB (2016) Physical and chemical changes of rapeseed meal proteins during toasting and their effects on in vitro digestibility. J Anim Sci Biotechnol. https://doi.org/10.1186/s40104-016-0120-x
Batal AB, Douglas MW, Engram AE, Parsons CM (2000) Protein dispersibility index as an indicator of adequately processed soybean meal. Poult Sci 79:1592–1596. https://doi.org/10.1093/ps/79.11.1592
Müller A, Wolf D, Gutzeit HO (2017) The black soldier fly, Hermetia illucens—a promising source for sustainable production of proteins, lipids and bioactive substances. Zeitschrift für Naturforschung C 72:351–363. https://doi.org/10.1515/znc-2017-0030
Firmansyah M, Yusuf M (2019) Heliyon production of protein hydrolysate containing antioxidant activity from Hermetia illucens. Heliyon 5:e02005. https://doi.org/10.1016/j.heliyon.2019.e02005
Rabani V, Cheatsazan H, Davani S (2019) Proteomics and lipidomics of Black Soldier Fly (Diptera: Stratiomyidae) and Blow Fly (Diptera: Calliphoridae) larvae. J Insect Sci. https://doi.org/10.1093/jisesa/iez050
Hall FG, Jones OG, Haire MEO, Liceaga AM (2017) Functional properties of tropical banded cricket (Gryllodes sigillatus) protein hydrolysates. Food Chem 224:414–422. https://doi.org/10.1016/j.foodchem.2016.11.138
Janssen RH, Vincken J, Arts NJG, Fogliano V, Lakemond CMM (2019) Effect of endogenous phenoloxidase on protein solubility and digestibility after processing of Tenebrio molitor, Alphitobius diaperinus and Hermetia illucens. Food Res Int 121:684–690. https://doi.org/10.1016/j.foodres.2018.12.038
Leni G, Soetemans L, Jacobs J, Depraetere S, Gianotten N, Bastiaens L, Caligiani A, Sforza S (2020) Protein hydrolysates from Alphitobius diaperinus and Hermetia illucens larvae treated with commercial proteases. J Insects Food Feed. https://doi.org/10.3920/jiff2019.0037
Zielińska E, Baraniak B, Karaś M (2017) Antioxidant and anti-inflammatory activities of hydrolysates and peptide fractions obtained by enzymatic hydrolysis of selected heat-treated edible insects. Nutrients 9:970. https://doi.org/10.3390/nu9090970
Shumo M, Osuga IM, Khamis FM, Tanga CM, Fiab KKM, Subramanian S, Ekesi S, Van Huis A, Borgemeister C (2019) The nutritive value of black soldier fly larvae reared on common organic waste streams in Kenya. Sci Rep. https://doi.org/10.1038/s41598-019-46603-z
Waśko A, Bulak P, Polak-berecka M, Nowak K, Polakowski C, Bieganowski A (2016) The first report of the physicochemical structure of chitin isolated from Hermetia illucens. Int J Biol Macromol 92:316–320. https://doi.org/10.1016/j.ijbiomac.2016.07.038
Konietzny U, Greiner R (2003) Phytic acid: nutritional impact. In: Caballero B, Trugo L, Finglas P (eds) Encyclopedia of food science and nutrition. Elsevier, London, pp 4555–4563
Kumar V, Sinha AK (2018) Chapter 3. General aspects of phytases, enzymes in human and animal nutrition. Elsevier Inc. https://doi.org/10.1016/B978-0-12-805419-2.0000. https://doi.org/10.1016/j.foodcont.2019.106877
Acknowledgements
Harish Karthikeyan Ravi is thankful to Region Sud PACA for the PhD grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Compliance with ethics requirements
This article does not contain any studies with human or animal subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ravi, H.K., Degrou, A., Costil, J. et al. Effect of devitalization techniques on the lipid, protein, antioxidant, and chitin fractions of black soldier fly (Hermetia illucens) larvae. Eur Food Res Technol 246, 2549–2568 (2020). https://doi.org/10.1007/s00217-020-03596-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-020-03596-8