Abstract
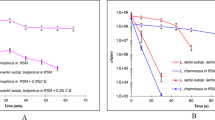
High hydrostatic pressure (HHP) processing of food can inactivate spoilage microorganisms, while largely preserving quality characteristics like color, flavor, texture, and vitamin content. However, the composition of the food matrix can strongly affect HHP inactivation efficiency. As systematic data on the interplay of different food matrix components are rare, we used defined oil-in-water (O/W) emulsions as a model system to investigate the impact of fat on the effect of the aqueous phase parameters sodium chloride (NaCl), sucrose, pH, and proteins on HHP inactivation of Lactobacillus (L.) plantarum strains. To identify a potential interconnection with cell surface characteristics, strains with hydrophobic and hydrophilic surface characteristics were considered. HHP inactivation varied widely among the investigated strains. In general, high NaCl and sucrose concentrations exerted a baroprotective effect, whereas low-pH environments enhanced inactivation, and the impact of proteins followed no clear trend. The presence of fat did not generally influence any of the aqueous phase parameters in its impact on HHP inactivation, but caused sporadic matrix and strain specific; however, cell surface hydrophobicity-independent alterations in inactivation efficiency. Still, fat was able to significantly change, and sometimes even invert, the effect of other food matrix parameters.








Similar content being viewed by others
References
Torres Bello EF, González Martínez G, Klotz Ceberio BF, Rodrigo D, Martínez López A (2014) High pressure treatment in foods. Foods 3(3):476–490. https://doi.org/10.3390/foods3030476
Cheftel JC (1995) Review : High-pressure, microbial inactivation and food preservation/Revisión: Alta-presión, inactivación microbiológica y conservación de alimentos. Food Sci Technol Int 1(2–3):75–90. https://doi.org/10.1177/108201329500100203
Smelt JPPM (1998) Recent advances in the microbiology of high pressure processing. Trends Food Sci Technol 9(4):152–158. https://doi.org/10.1016/S0924-2244(98)00030-2
Considine KM, Kelly AL, Fitzgerald GF, Hill C, Sleator RD (2008) High-pressure processing—effects on microbial food safety and food quality. FEMS Microbiol Lett 281(1):1–9. https://doi.org/10.1111/j.1574-6968.2008.01084.x
van de Ven C, Courvoisier C, Matser A (2007) High pressure versus heat treatments for pasteurisation and sterilisation of model emulsions. Innovat Food Sci Emerg Technol 8(2):230–236. https://doi.org/10.1016/j.ifset.2006.12.001
Cebrián G, Mañas P, Condón S (2016) Comparative resistance of bacterial foodborne pathogens to non-thermal technologies for food preservation. Front Microbiol 7:734. https://doi.org/10.3389/fmicb.2016.00734
Patterson MF (2005) Microbiology of pressure-treated foods. J Appl Microbiol 98(6):1400–1409. https://doi.org/10.1111/j.1365-2672.2005.02564.x
Oxen P, Knorr D (1993) Baroprotective effects of high solute concentrations against inactivation of Rhodotorula rubra. LWT Food Sci Technol 26(3):220–223
Molina-Höppner A, Doster W, Vogel RF, Gänzle MG (2004) Protective effect of sucrose and sodium chloride for Lactococcus lactis during sublethal and lethal high-pressure treatments. Appl Environ Microbiol 70(4):2013–2020
Simpson RK, Gilmour A (1997) The effect of high hydrostatic pressure on Listeria monocytogenes in phosphate-buffered saline and model food systems. J Appl Microbiol 83(2):181–188. https://doi.org/10.1046/j.1365-2672.1997.00215.x
Van Opstal I, Vanmuysen SCM, Michiels CW (2003) High sucrose concentration protects E. coli against high pressure inactivation but not against high pressure sensitization to the lactoperoxidase system. Int J Food Microbiol 88(1):1–9. https://doi.org/10.1016/S0168-1605(03)00070-9
Narisawa N, Furukawa S, Kawarai T, Ohishi K, Kanda S, Kimijima K, Negishi S, Ogihara H, Yamasaki M (2008) Effect of skimmed milk and its fractions on the inactivation of Escherichia coli K12 by high hydrostatic pressure treatment. Int J Food Microbiol 124(1):103–107. https://doi.org/10.1016/j.ijfoodmicro.2008.01.016
Black EP, Huppertz T, Fitzgerald GF, Kelly AL (2007) Baroprotection of vegetative bacteria by milk constituents: a study of Listeria innocua. Int Dairy J 17(2):104–110. https://doi.org/10.1016/j.idairyj.2006.01.009
Stewart CM, Jewett FF, Dunne CP, Hoover DG (1997) Effect of concurrent high hydrostatic pressure, acidity and heat on the injury and destruction of Listeria monocytogenes. J Food Saf 17(1):23–36. https://doi.org/10.1111/j.1745-4565.1997.tb00173.x
Garcia-Graells C, Hauben EJA, Michiels CW (1998) High-pressure inactivation and sublethal injury of pressure-resistant Escherichia coli mutants in fruit juices. Appl Environ Microbiol 64(4):1566–1568
Alpas H, Kalchayanand N, Bozoglu F, Ray B (2000) Interactions of high hydrostatic pressure, pressurization temperature and pH on death and injury of pressure-resistant and pressure-sensitive strains of foodborne pathogens. Int J Food Microbiol 60(1):33–42. https://doi.org/10.1016/S0168-1605(00)00324-X
Ritz M, Jugiau F, Rama F, Courcoux P, Semenou M, Federighi M (2000) Inactivation of Listeria monocytogenes by high hydrostatic pressure: effects and interactions of treatment variables studied by analysis of variance. Food Microbiol 17(4):375–382. https://doi.org/10.1006/fmic.2000.0336
Arroyo C, Cebrián G, Mackey BM, Condón S, Pagán R (2011) Environmental factors influencing the inactivation of Cronobacter sakazakii by high hydrostatic pressure. Int J Food Microbiol 147(2):134–143. https://doi.org/10.1016/j.ijfoodmicro.2011.03.018
Li H, Garcia-Hernandez R, Driedger D, McMullen LM, Gänzle M (2016) Effect of the food matrix on pressure resistance of Shiga-toxin producing Escherichia coli. Food Microbiol 57:96–102. https://doi.org/10.1016/j.fm.2016.02.002
Bover-Cid S, Belletti N, Aymerich T, Garriga M (2015) Modeling the protective effect of aw and fat content on the high pressure resistance of Listeria monocytogenes in dry-cured ham. Food Res Int 75:194–199. https://doi.org/10.1016/j.foodres.2015.05.052
Bover-Cid S, Belletti N, Aymerich T, Garriga M (2017) Modelling the impact of water activity and fat content of dry-cured ham on the reduction of Salmonella enterica by high pressure processing. Meat Sci 123:120–125. https://doi.org/10.1016/j.meatsci.2016.09.014
Escriu R, Mor-Mur M (2009) Role of quantity and quality of fat in meat models inoculated with Listeria innocua or Salmonella Typhimurium treated by high pressure and refrigerated stored. Food Microbiol 26(8):834–840. https://doi.org/10.1016/j.fm.2009.05.011
Ramaswamy HS, Jin H, Zhu S (2009) Effects of fat, casein and lactose on high-pressure destruction of Escherichia coli K12 (ATCC-29055) in milk. Food Bioprod Process 87(1):1–6. https://doi.org/10.1016/j.fbp.2008.02.005
Gervilla R, Ferragut V, Guamis B (2000) High pressure inactivation of microorganisms inoculated into bovine milk of different fat contents. J Dairy Sci 83(4):674–682. https://doi.org/10.3168/jds.S0022-0302(00)74928-9
Kruk ZA, Kim HJ, Kim YJ, Rutley DL, Jung S, Lee SK, Jo C (2014) Combined effects of high pressure processing and addition of soy sauce and olive oil on safety and quality characteristics of chicken breast meat. Asian-Aust J Anim Sci 27(2):256–265. https://doi.org/10.5713/ajas.2013.13417
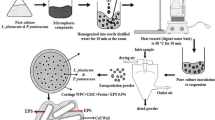
Kafka TA, Reitermayer D, Lenz CA, Vogel RF (2017) High hydrostatic pressure inactivation of Lactobacillus plantarum cells in (O/W)-emulsions is independent from cell surface hydrophobicity and lipid phase parameters. High Pressure Res 37(3):430–448. https://doi.org/10.1080/08957959.2017.1347926
Kafka TA (2018) Role of lipid phase composition and cell surface hydrophobicity in high pressure inactivation of Lactobacillus plantarum in emulsions. Dissertation, Technische Universität München, Freising
Smittle RB (1977) Microbiology of mayonnaise and salad dressing: a review. J Food Prot 40(6):415–422. https://doi.org/10.4315/0362-028x-40.6.415
Goldstein EJC, Tyrrell KL, Citron DM (2015) Lactobacillus species: Taxonomic complexity and controversial susceptibilities. Clin Infect Dis 60(suppl 2):S98–S107. https://doi.org/10.1093/cid/civ072
Liévin-Le Moal V, Servin AL (2014) Anti-infective activities of Lactobacillus strains in the human intestinal microbiota: from probiotics to gastrointestinal anti-infectious biotherapeutic agents. Clin Microbiol Rev 27(2):167–199. https://doi.org/10.1128/cmr.00080-13
Guidone A, Zotta T, Ross RP, Stanton C, Rea MC, Parente E, Ricciardi A (2014) Functional properties of Lactobacillus plantarum strains: a multivariate screening study. LWT Food Sci Technol 56(1):69–76. https://doi.org/10.1016/j.lwt.2013.10.036
de Vries MC, Vaughan EE, Kleerebezem M, de Vos WM (2006) Lactobacillus plantarum—survival, functional and potential probiotic properties in the human intestinal tract. Int Dairy J 16(9):1018–1028. https://doi.org/10.1016/j.idairyj.2005.09.003
Hammes WP, Bantleon A, Min S (1990) Lactic acid bacteria in meat fermentation. FEMS Microbiol Lett 87(1–2):165–174. https://doi.org/10.1111/j.1574-6968.1990.tb04886.x
Siezen RJ, van Hylckama Vlieg JET (2011) Genomic diversity and versatility of Lactobacillus plantarum, a natural metabolic engineer. Microb Cell Fact 10(Suppl 1):S3–S3. https://doi.org/10.1186/1475-2859-10-S1-S3
McDonald LC, Fleming HP, Hassan HM (1990) Acid tolerance of Leuconostoc mesenteroides and Lactobacillus plantarum. Appl Environ Microbiol 56(7):2120–2124
Cebeci A, Gürakan C (2003) Properties of potential probiotic Lactobacillus plantarum strains. Food Microbiol 20(5):511–518. https://doi.org/10.1016/S0740-0020(02)00174-0
G-alegría E, López I, Ruiz JI, Sáenz J, Fernández E, Zarazaga M, Dizy M, Torres C, Ruiz-Larrea F (2004) High tolerance of wild Lactobacillus plantarum and Oenococcus oeni strains to lyophilisation and stress environmental conditions of acid pH and ethanol. FEMS Microbiol Lett 230(1):53–61. https://doi.org/10.1016/S0378-1097(03)00854-1
Fernández Ramírez MD, Smid EJ, Abee T, Nierop Groot MN (2015) Characterisation of biofilms formed by Lactobacillus plantarum WCFS1 and food spoilage isolates. Int J Food Microbiol 207:23–29. https://doi.org/10.1016/j.ijfoodmicro.2015.04.030
Sanders JW, Oomes SJCM, Membré JM, Wegkamp A, Wels M (2015) Biodiversity of spoilage lactobacilli: phenotypic characterisation. Food Microbiol 45(Part A):34–44. https://doi.org/10.1016/j.fm.2014.03.013
Kurtzman CP, Rogers R, Hesseltine CW (1971) Microbiological spoilage of mayonnaise and salad dressings. Appl Microbiol 21(5):870–874
Chenoll E, Macián MC, Elizaquível P, Aznar R (2007) Lactic acid bacteria associated with vacuum-packed cooked meat product spoilage: population analysis by rDNA-based methods. J Appl Microbiol 102(2):498–508. https://doi.org/10.1111/j.1365-2672.2006.03081.x
Lyhs U, Korkeala H, Vandamme P, Björkroth J (2001) Lactobacillus alimentarius: a specific spoilage organism in marinated herring. Int J Food Microbiol 64(3):355–360. https://doi.org/10.1016/S0168-1605(00)00486-4
Matamoros S, André S, Hue I, Prévost H, Pilet MF (2010) Identification of lactic acid bacteria involved in the spoilage of pasteurized “foie gras” products. Meat Sci 85(3):467–471. https://doi.org/10.1016/j.meatsci.2010.02.017
Lundstrom HS, Bjorkroth J (2007) Lactic acid bacteria in marinades used for modified atmosphere packaged broiler chicken meat products. J Food Prot 70(3):766–770. https://doi.org/10.4315/0362-028x-70.3.766
Lenz CA, Vogel RF (2014) Effect of sporulation medium and its divalent cation content on the heat and high pressure resistance of Clostridium botulinum type E spores. Food Microbiol 44:156–167
Lenz CA, Reineke K, Knorr D, Vogel RF (2015) High pressure thermal inactivation of Clostridium botulinum type E endospores–kinetic modeling and mechanistic insights. Front Microbiol 6:652. https://doi.org/10.3389/fmicb.2015.00652
Alpas H, Kalchayanand N, Bozoglu F, Sikes A, Dunne CP, Ray B (1999) Variation in resistance to hydrostatic pressure among strains of food-borne pathogens. Appl Environ Microbiol 65(9):4248–4251
Pagan R, Mackey B (2000) Relationship between membrane damage and cell death in pressure-treated Escherichia coli cells: differences between exponential- and stationary-phase cells and variation among strains. Appl Environ Microbiol 66(7):2829–2834. https://doi.org/10.1128/aem.66.7.2829-2834.2000
Koseki S, Yamamoto K (2006) pH and solute concentration of suspension media affect the outcome of high hydrostatic pressure treatment of Listeria monocytogenes. Int J Food Microbiol 111(2):175–179. https://doi.org/10.1016/j.ijfoodmicro.2006.05.008
Acknowledgements
Part of this work was supported by the German Ministry of Economics and Technology (via AiF) and the FEI (Forschungskreis der Ernährungsindustrie e.V. Bonn) in project AiF 17463N.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Reitermayer, D., Kafka, T.A., Lenz, C.A. et al. Interaction of fat and aqueous phase parameters during high-hydrostatic pressure inactivation of Lactobacillus plantarum in oil-in-water emulsions. Eur Food Res Technol 246, 1269–1281 (2020). https://doi.org/10.1007/s00217-020-03487-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-020-03487-y