Abstract
Edible fruits are known as source of bioactive compounds, however, growing interest in the use of plant byproducts has been observed in last few years. The objective of study was to compare the chemical composition, fatty acid profile, content of bioactive compounds, including the HPLC analysis of anthocyanins and antioxidant activity of sweet cherry fruit, petioles and leaves of the following cultivars: Burlat, Kordia and Regina. In the fruit, the major fatty acid was oleic acid, and in the petioles—palmitic acid and in the leaves—the γ-linolenic acid. The petioles were characterized by the highest antioxidant activity and content of polyphenols, whereas the anthocyanins were detected only in fruit. Two anthocyanins were identified: cyanidin 3-glucoside and cyanidin 3-rutinoside. Fruit of cultivar Kordia as well as petioles and leaves of cultivars Burlat and Regina had the highest antioxidant activity. There is a need for further research (especially in vivo studies). This knowledge can be used to create a new functional food and to better use of byproducts of sweet cherry production.
Similar content being viewed by others
Introduction
Fruits and vegetables are a very important part of daily diet. Eating 400–500 g or five portions of fruits and vegetables per day is recommended by WHO [1, 2]. To increase intake of this products, we should always include vegetables in meals, eat fresh fruits and raw vegetables in season as well as choose them as snacks [3].
Many studies indicated that consumption of fruits is associated with reduced risk of non-communicable diseases, especially cardiovascular diseases as well as some types of cancers, such as colorectal, pharynx, larynx, lung, esophagus, stomach and cervix uterus cancers [4]. Health-promoting potential of fruits is due to a significant content of bioactive compounds, especially antioxidants, which may scavenge free radicals, inhibit pro-oxidative enzymes (i.a. lipoxygenase, myeloperoxidase) and bind of metal ions (such as iron), thus protect against oxidation of lipids and proteins as well as atherosclerosis and heart diseases [5, 6]. The available literature showed that the fruit of sweet cherry contains significant amounts of polyphenols (including anthocyanins), vitamin C, dietary fiber as well as potassium and zinc [7, 8]. A number of in vitro studies have shown that the extracts of sweet cherry fruit possess strong antioxidant activity (mainly due to high polyphenols content), may reduce the risk of cancer (especially colon cancer) as well as cardiovascular and neurodegenerative diseases [9,10,11].
Food producers do not take into account other than fruit parts of shrubs growing in the wild or fruit trees, meanwhile, studies have shown that the leaves of these plants are an excellent source of bioactive components [12,13,14]. In the available literature, there is little of data on the chemical composition as well as content of bioactive compounds in the petioles and leaves of sweet cherry; therefore, it is reasonable to assess not only the fruit but also other part of sweet cherry.
The objective of this study was to compare the basic chemical composition, fatty acid profile, content of bioactive compounds, including the HPLC analysis of anthocyanins as well as antioxidant activity of fruit, petioles and leaves of three cultivars of sweet cherry.
Materials and methods
Reagents
All the chemicals were of analytical grade. Potassium persulfate, hydrochloric acid, ferric chloride hexahydrate, oxalic acid, sulfuric acid, sodium sulfide, mercury chloride, acetone and n-hexane were obtained from Chempur (Piekary Śląskie, Poland), methanol from POCh (Katowice, Poland), chlorogenic acid, Folin–Ciocalteu reagent, ABTS (2,2′-Azino-bis(3-ethylbenzthiazoline-6-sulfonic acid)), DPPH (2,2-diphenyl-1-picrylhydrazyl) and TPTZ (2,4,6- tris(2-pyridyl)-s-triazine) from Sigma-Aldrich (Saint Louis, MO, USA) as well as cyanidin 3-rutinoside from Polyphenols AS (Sandnes, Norway).
Plant material
The fruit, petioles and leaves of sweet cherry were collected in 2015 and 2016 from the Experimental Station of Pomology and Apiculture Department, in Garlica Murowana, University of Agriculture in Krakow (Poland). The study included the three cultivars of sweet cherry: Burlat, Kordia and Regina. The fruit together with petioles and leaves was randomly picked at commercial maturity of fruit: cultivar Burlat in the fourth week of June 2015 and in the first week of July 2016, cultivar Kordia in the third week of July 2015 and 2016 as well as cultivar Regina in the fourth week of July 2015 and 2016. In the fresh material, the level of vitamin C was determined. Additionally, fresh fruit, petioles and leaves were used for the preparation of methanolic extracts. Other analyzes were carried out on the freeze-dried samples. To prepare material for freeze drying, the fruit was cut in half and pitted. The half of fruit, petioles and leaves were transferred to plastic plate and frozen at − 80 °C. Next, the samples with the plates were placed in a freeze dryer [Christ Alpha 1–4 (Osterode am Harz, Germany)]. The process was continued for 48 h for fruit and 24 h for petioles and leaves. After freeze drying, the samples were tightly closed in a plastic bag to protect from moisture.
Basic chemical composition
In freeze–dried fruit, petioles and leaves, the basic chemical composition was determined using the AOAC methods [15]. The content of total proteins was measured according to procedure no. 950.36, crude fat—procedure no. 935.38 and ash—procedure no. 930.05. The level of dietary fiber was measured by commercially available kit (cat no. K-TDFR-100A, Megazyme International Ireland, Wicklow, Ireland). The digestible carbohydrate level was calculated with the using of equation: digestible carbohydrates = 100 − (protein + crude fat + ash + dietary fiber) [16].
Fatty acid profile
The fatty acid profile was assessed by gas chromatography after extraction of lipids from the fruit, petioles and leaves of sweet cherry using the Folch’s method [17]. The free fatty acids were transformed into their respective methylated derivatives (methyl esters) in 14% (v/v) BF3/Me–OH and extracted using hexane as fatty acids methyl esters and separated with the GC-17A-QP5050 GC-MS model (Shimadzu, Kioto, Japan), using the capillary SP-2560 column (30 m × 0.25 mm × 0.25 µm; Supelco, Bellefonte, PA, USA). The carrier gas was helium with the flow rate of 5 ml/min. The temperature of column was kept at 60 °C for 5 min and then increased up to 220 °C (5 ºC/min). This temperature was maintained for 23 min.
Extract preparation
The extracts were prepared by shaking of 1 ± 0.005 g of fresh sweet cherry fruit or 0.5 ± 0.003 g petioles and leaves in 80 ml of 70% methanol acidified HCl at room temperature for 2 h (Elpin Plus, water bath shaker type 357, Lubawa, Poland). Then the samples were centrifuged at 1500 rpm for 15 min (Centrifuge type MPW-340, Warsaw, Poland). Supernatants were stored at temperature − 20 °C until analysis.
Determination of selected bioactive compounds
The vitamin C content was measured in fresh material as sum of ascorbic and dehydroascorbic acid with using Tillmans method, as modified by Pijanowski [16]. 10 ± 0.008 g of fruit as well as 5 ± 0.005 g of petioles and leaves were triturated using 2% oxalic acid in a porcelain mortar, then transferred to a 100-ml volumetric flask and made up to mark with 2% oxalic acid. The mixture was filtered. 10 ml of the filtrate was transferred to a 50-ml volumetric flask. Then, 3.5 ml of 0.5M sulfuric acid and 1.75 ml of 1.0M sodium sulfide were added. The solution was left for 10 min. After this time, 2.5 ml of 1.0 M mercury chloride was added. The content of the flask was mixed and made up to the mark with distilled water. The solution was filtered. 10 ml of the filtrate was 2,6-dichlorophenolindophenol titrated. The content of total carotenoids was determined spectrophotometrically by extracting carotenoids from the freeze–dried samples using acetone–hexane mixture (4:6 v/v) according to the PN-EN 12136:2000. The absorbance was measured at 450 nm using the spectrophotometer (UV-1800, RayLeigh, Beijing Beifen-Ruili Analytical Instrument Co., Ltd., Beijing, China). The content of total polyphenols was estimated spectrophotometrically in methanolic extracts with using the Folin–Ciocalteu reagent as previously reported [18]. The concentration of total anthocyanins was also studied spectrophotometrically in methanolic extracts by the pH differential methods, using potassium chloride buffer, pH 1.0 (0.025 M) and sodium acetate buffer, pH 4.5 (0.4 M) [19]. The absorbance was measured at 510 nm and 700 nm (spectrophotometer UV-1800, RayLeigh, Beijing Beifen-Ruili Analytical Instrument Co., Ltd., Beijing, China).
HPLC analysis of anthocyanins
The HPLC analysis of anthocyanins was carried out on samples collected in 2016, according to modified method described by Goiffon et al. [20]. Analysis was performed using a 1200 series liquid chromatograph manufactured by Agilent Technologies (Palo Alto, CA, USA), equipped with a diode array detector (DAD) from the same manufacturer and LCMS-2010EV detector (Shimadzu, Kyoto, Japan). The separation was performed on a column with modified silica gel packing LiChrospher C18 (250 × 4.6 mm), particle size 5 µm (Merck KGaA, Darmstadt, Germany), at 30 °C. The mobile phase was a mixture of solvents: water:acetonitrile:formic acid (81:9:10 v/v/v). The flow rate of the mobile phase was 1 ml/min. The amount of injected solution was 10 µl. Identification of separated anthocyanins was based on MS spectra. The content of analyzed compounds was calculated based on the cyanidin 3-rutinoside calibration curve with DAD detector set at a wavelength of 520 nm.
Determination of antioxidant activity
The antioxidant activity was measured using three methods: ABTS (2,2′-Azino-bis(3-ethylbenzthiazoline-6-sulfonic acid)), DPPH (2,2-diphenyl-1-picrylhydrazyl) and FRAP (ferric reducing antioxidant power).
ABTS method
The determination of antioxidant activity using the free radical ABTS•+ was estimated as previously reported by Re et al. [21] with minor modifications. The working solution was obtained by diluting the ABTS•+ stock solution with 70% methanol to obtain the absorbance of 0.740–0.750 at 734 nm. A 10–200 µl of the extract [so that RSA (radical scavenging activity) was 50–70%] were transferred into a test tube and made up to 1 ml with 70% methanol. After mixing of a diluted extract with 2 ml of working solution of the ABTS•+, the mixture was kept in dark at 30 °C for 6 min.
RSA was calculated using the equation: RSA (%) = [(AABTS − AS)/AABTS] × 100, where AABTS is the absorbance of the ABTS working solution, and AS is the absorbance of the sample solution.
DPPH method
The DPPH assay was done according to Miliauskas et al. [22] with some modifications. The stock solution of DPPH• was prepared by dissolving 6 mg of 2,2-Diphenyl-1-picrylhydrazyl in 100 ml of methanol. The working solution was obtained by diluting the stock solution with methanol to obtain the absorbance of 0.900–1.000 at 515 nm. A 10–200 µl of the extract were transferred into a test tube and made up to 1.5 ml with methanol. After mixing of a diluted extract with 3 ml of solution of the DPPH•, the mixture was kept in dark at room temperature for 10 min.
FRAP method
The measure of the total reducing capability, using FRAP method, was determined as previously reported by Benzie and Strain [23] with adaptations. A 10–200 µl of the extract were transferred into a test tube and made up to 1 ml with 70% methanol. After mixing of a diluted extract with 3 ml of working solution of the FRAP reagent, the mixture was kept in dark at room temperature for 10 min.
The absorbance of the samples was measured at 734 nm in ABTS, 515 nm in DPPH and 593 nm in FRAP method [spectrophotometer (UV-1800, RayLeigh, Beijing Beifen-Ruili Analytical Instrument Co., Ltd., Beijing, China)]. The obtained values were expressed as micromoles of Trolox equivalent per gram of sample (TEAC).
Statistical analysis
Statistical analysis was conducted with the program Statistica v. 13.1 (Tulsa, OK, USA). All assays were done in triplicate. Results were expressed as the means ± standard deviation (SD). Data were analyzed using multiple-way analysis of variance (ANOVA), including the three factors: cultivar, part of plant and harvested year as fixed effects on chemical composition of sweet cherry. Exception was statistical analyze of content of total anthocyanins which was found only in fruits (two-way ANOVA), level of individual anthocyanins which was determined only in 2016 (one-way ANOVA) as well as concentration of fatty acids (one-way ANOVA). Significant differences between mean values were compared using Duncan’s test. Correlations between the tested features were examined using Pearson correlation. P values < 0.05 were regarded as significant.
Meteorological data
Average daily and monthly temperature as well as rainfall in 2015 and 2016 was obtained from Agrometeorological Station of Department of Ecology, Climatology and Air Protection in Garlica Murowana, University of Agriculture in Krakow (Poland).
Results
Basic chemical composition
The fruit of sweet cherry was characterized by the significantly highest content of digestible carbohydrates as well as the lowest content of ash, protein (excluding cultivar Burlat from 2015), crude fat and dietary fiber in comparison to other parts of plant of all cultivars (Table 1). Meanwhile, the leaves were the richest in protein and the poorest in digestible carbohydrates, which was confirmed in all cultivars. The leaves were also the best source of dietary fiber but exception was the cultivar Kordia and Regina collected in 2015, where the highest content of dietary fiber was found in the petioles. Among the fruit, the cultivar Kordia from 2015 was characterized by the highest content of ash, however, from 2016 by the highest level of protein and crude fat in comparison to other cultivars. Statistically significant effect of cultivar on the level of fiber and digestible carbohydrates in fruit from both years was not found. Among the petioles, the cultivar Burlat from 2015 was characterized by the highest content of digestible carbohydrates, the cultivar Kordia from both years—the largest amount of ash and dietary fiber as well as cultivar Regina from both years—the highest level of protein, in comparison to other cultivars. Among the leaves, cultivar Burlat from 2015 was the richest in fiber as well as cultivars Kordia and Regina were one of the better sources of ash and digestible carbohydrates in 2015 and 2016. In the most cases, the basic chemical composition of assessed sweet cherry fruit, petioles and leaves of individual cultivars varied with harvest years.
Fatty acid profile
In the fruit, petioles and leaves of sweet cherry, the following fatty acids have been identified: palmitic acid, stearic acid, oleic acid, linoleic acid and γ-linolenic acid (Table 2). In addition, in the petioles and leaves, eicosapentaenoic acid as well as behenic acid were found. In the fruit, the dominant fatty acid was oleic acid. The cultivar Kordia from 2015 and Regina from both years were the richest source of this compound. In all the studied petioles, the main fatty acid was palmitic acid. The cultivar Burlat from 2015 was the best source of this component. In leaves, the most numerous fatty acids were γ-linolenic acid. The cultivar Kordia and Regina from 2016 was characterized by the highest content of this compound. Generally, the level of individual fatty acids in samples was different with harvest years.
The bioactive compounds
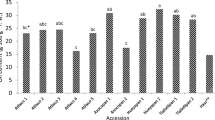
The significantly lowest level of bioactive compounds was determined in fruits of all cultivars in comparison to other parts of sweet cherry (Table 3). The exception was the anthocyanin content which was identified only in fruit (Table 3). The leaves were characterized by the highest level of vitamin C and total carotenoids as compared to other parts of plant. Moreover, the petioles had the highest content of total polyphenols. Among the fruit from 2015, the cultivar Burlat was characterized by the significantly largest amount of total carotenoids, meanwhile from 2016—the cultivar Kordia in comparison to other cultivars. Moreover, the highest concentration of total polyphenols was in fruit of cultivars Kordia from both years. The petioles of cultivar Burlat were one of the best source of vitamin C and polyphenolic compounds, whereas cultivar Kordia—total carotenoids, in both years. The leaves of cultivar Kordia from 2016 had the significantly highest content of total carotenoids, while cultivar Regina from 2016—vitamin C and total polyphenols in comparison to other cultivars. The significantly largest amount of total anthocyanins was in fruit of cultivar Burlat from 2015 and cultivar Kordia from both years. In HPLC analysis, two anthocyanins were identified: cyanidin 3-glucoside and cyanidin 3-rutinoside (Fig. 1). The fruit of cultivar Kordia was characterized by the significantly highest content of both identified anthocyanins (respectively, 3.2 ± 0.1 and 49.1 ± 0.1 mg/100 g DW). The significantly lowest level of these compounds was found in cultivar Regina in comparison to other cultivars (respectively, 1.2 ± 0.1 and 25.6 ± 1.7 mg/100 g DW). Fruit of cultivar Burlat contained 2.3 ± 0.1 mg/100 g DW of cyanidin 3-glucoside and 33.8 ± 0.9 mg/100 g DW of cyanidin 3-rutinoside. The results indicated that major anthocyanin was cyanidin 3-rutinoside. Generally, the level of bioactive compounds varies depending on the year of cultivation. It has been observed that in most cases the content of studied components was higher in 2016 than in 2015.
The antioxidant activity
The significantly lowest antioxidant activity were found in fruit, whereas the largest in petioles of sweet cherry which was confirmed in all cultivars both in 2015 and 2016 (Table 4). Among the fruit from 2015 to 2016, the cultivar Kordia was characterized by the significantly highest antioxidant activity. The petioles of cultivars Kordia (ABTS assay) and Regina (DPPH and FRAP assays) had the highest antioxidant capacity in 2015. Moreover, the petioles of cultivar Burlat were characterized by the highest antioxidant activity in 2016 which was confirmed by ABTS, DPPH and FRAP tests. Among the leaves from 2015, the cultivar Burlat, and from 2016, the cultivar Regina had the significantly highest radical scavenging activity in comparison to other cultivars. The results obtained in this study showed that the antioxidant activity in samples was higher in 2016.
Discussion
To our best knowledge, there are no reports on the basic chemical composition of sweet cherry petioles and leaves. However, the content of macronutrient in fruit was previously analyzed by other authors. De Souza et al. [24] showed the similar level of ash, protein and carbohydrates as well as the slightly higher content of fat in sweet cherry fruit from São Paulo in comparison to the ones obtained in this study. The fruit from Northern Portugal examined by Bastos et al. [9] as well as from Southern Italy tested by Pacifico et al. [11] had the lower content of ash, protein and fat as well as the higher content of carbohydrates. In our study, the concentration of dietary fiber in the fruit of sweet cherry was in the range of 7.0 ± 0.0–10.8 ± 0.3 g/100 g DW. De Souza et al. [24] found higher content of dietary fiber in these parts of plant (15.25 g/100 g DW).
In the literature, there is little research on the profile of fatty acids in sweet cherry. Rios et al. [25], who also analyzed the fruit of cultivar Kordia and Regina, originating from Chile, found that the dominant fatty acid was palmitic acid. These data correspond to our results. According to Bastos et al. [9] in the petioles, the main fatty acid was palmitic acid, whereas according to Schmitt and Feucht [26] in the leaves, the dominant fatty acid was γ-linolenic acid, which are consistent with our results.
In fruit, the level of vitamin C was in the range of 28.2 ± 0.0–51.5 ± 3.2 mg/100 g DW. The similar results were obtained by Schmitz-Eiberger and Blanke [27] in the following cultivars: Samba, Souvenir de Charmes and Prime Giant. De Souza et al. [24] showed the higher content of vitamin C. Usenik et al. [28] who evaluated the effect of diameter and color of fruit on chemical composition in cultivar Kordia found the lower concentration of vitamin C in each quality classes, in comparison to values obtained for cultivar Kordia in our study. The content of total carotenoids in tested fruit of sweet cherry was in the range of 0.2 ± 0.0–3.8 ± 0.0 mg/100 g DW. The level of these compounds measured by Giménez et al. [10] in fruit from Spain was 1.07 mg/100 g DW. Gonçalves et al. [29] showed the higher concentration of total carotenoids in the leaves of three cultivars of sweet cherry, including the cultivar Burlat which was also examined in this study. The differences are probably due to weather conditions in the harvest season. The literature data indicated that the concentration of bioactive compounds, including vitamin C, was known to be influenced by several factors such as temperature and precipitation [30]. To our best knowledge, in the literature, there is no data about the content of bioactive compounds in petioles and leaves, discussed above. Exception was the content of carotenoids in the leaves, as described above.
The obtained results showed that the content of total polyphenols in fruit of sweet cherry from 2015 to 2016 was in the range of 1987.8 ± 41.1–3063.1 ± 21.1 mg/100 g DW. De Souza et al. [24] found the similar values. Other authors obtained the lower concentration of polyphenolic compounds in sweet cherry fruit [27, 31, 32]. Ballistreri et al. [7] and Usenik et al. [33] who also analyzed the cultivar Burlat found the lower content of total polyphenols with respect to that reported in this study. In our study, the petioles were the richest source of polyphenolic compounds in comparison to other parts of sweet cherry. Prvulović et al. [34] showed the smaller amount of these compounds in petioles of cultivar Burlat and other 16 cultivars collected in Serbia. Di Cagno and Coda [35], who studied the infusions prepared from petioles, also demonstrated the lower content of total polyphenols in this part of sweet cherry. Gonçalves et al. [29] showed the similar polyphenols content in leaves of cultivar Burlat and lower in other cultivars in comparison to our results. Based on the results, it can be suggested that the petioles and leaves were a richer source of polyphenolic compounds than commonly consumed fruit of sweet cherry. Due to antioxidant, anti-inflammatory, anticancer and antibacterial activity of polyphenols, petioles and leaves should be included in the daily diet. They can be used for the preparation of various tea or food and beverages addition.
The concentration of total anthocyanins in sweet cherry fruit was studied by many authors. Hayaloglu and Demir [31] determined the similar content of these compounds in fruit of cultivar Belge and the lower content in other cultivars came from Turkey. The smaller amount of total anthocyanins was also found by Giménez et al. [10] in cultivar Early Lory and Schmitz-Eiberger and Blanke [27] in cultivars Souvenir, Samba and Prime Giant. The higher concentration of these compounds was obtained by Serradilla et al. [32] in cultivar Ambrunés from Jerte Valley, Spain. Other authors who analyzed the sweet cherry fruit using HPLC method detected four or more anthocyanins, and the dominant anthocyanin in all research was cyanidin 3-rutinoside, which correspond to our results. Serra et al. [36] found the similar content of cyanidin 3-glucoside and cyanidin 3-rutinoside in cultivar Summit. The fruit of cultivar Ambrunés, which was studied by Serradilla et al. [32], was characterized by the similar level of cyanidin 3-glucoside and higher content of cyanidin 3-rutinoside. Martini et al. [37] obtained the larger amounts of these two anthocyanins in different cultivars of sweet cherry fruit in comparison to our results. Ballistreri et al. [7] and Usenik et al. [33] who also tested the cultivar Burlat showed the higher content of cyanidin 3-glucoside and cyanidin 3-rutinoside in fruit of this cultivar. The differences in content of anthocyanins may result from various growing conditions. Fruit of sweet cherry analyzed by other authors was harvested in countries differed in climatic and geographical conditions.
Additionally, the studies were allowed to choose the fruit of cultivar Kordia, which was characterized by the highest level of polyphenols, including anthocyanins. The color of sweet cherry fruit, which is related to content of anthocyanins, is important factor determining the maturity and attractiveness. Consumers, who are guided by the color of the skin, reach for not only good look product but also with beneficial properties.
The antioxidant activity in samples was measured using three methods: ABTS, DPPH and FRAP. More than one test was needed to be performed to take into account the various modes of antioxidants action [38]. Used methods presented coherent results for the studied material. Serradilla et al. [32] who evaluated the radical scavenging activity of sweet cherry fruit showed the higher level in ABTS test in comparison to our results. Hayaloglu and Demir [31] who used the ABTS, DPPH and FRAP assays also found the higher values. The lower antioxidant activity in fruit of sweet cherry was reported by De Souza et al. [24]. The different values for antioxidant activity obtained by other authors may result from other method of extract preparation, differences and modifications in procedure for the ABTS, DPPH and FRAP assays as well as other cultivars used to research.
In the available literature, there were little research which compared the polyphenol content and antioxidant activity of various parts of sweet cherry. Bastos et al. [9] obtained other results and indicated that the fruit was characterized by higher antioxidant capacity than petioles of sweet cherry. These different results are probably due to other method of extract preparation. Other authors, who examined these properties in parts of other fruit trees and shrubs, showed the similar findings to ours. Skupień et al. [13] in chokeberry and mulberry as well as Teleszko and Wojdyło [14] in apple, quince, chokeberry, cranberry, blackcurrant and bilberry found that leaves of these plants were characterized by higher content of polyphenols and antioxidant activity than fruits.
The correlation between the content of bioactive compounds and antioxidant activity in studied material is presented in Table 5. In all tested parts of sweet cherry, the relationship between antioxidant capacity and dietary fiber content was weak and additionally in fruit and petioles was negative. The positive and strong dependency between antioxidant activity and the content of total polyphenols was found in all parts of plant. Furthermore, in fruit, the antioxidant activity strongly depended on vitamin C and total carotenoids content, while in petioles on the concentration of carotenoids. In summary, polyphenols were found to be main antioxidant components of all samples, whereas in particular parts of sweet cherry, i.e., fruit, petioles and leaves, there were also other compounds responsible for antioxidant activity.
In general, the content of studied compounds differs from one harvest year to another one. The average temperature in April (beginning of flowering), May and June 2016 was higher than in the same months in 2015, while in the July (harvest period) 2016 lower than in 2015 (Fig. 2). The number of rainy days and the sum of precipitation in April and July 2016 were higher than in 2015, however, in May and June 2016, lower (Fig. 3). Therefore, it could be assumed that a temperature and precipitation has an important effect on the chemical composition of sweet cherry fruit, petioles and leaves. To our best knowledge, there is no study about the effect of weather conditions on content of individual compounds in the sweet cherry. Moreover, other authors who studied other plant confirmed these observations. Hakala et al. [39] who studied the content of vitamin C in strawberries reported the higher content of this compound in the year in which the summer was warmer and drier which is consistent with our results. However, Woznicki et al. [40] found that the level of vitamin C in black currant fruits was negatively correlated with temperature and positively correlated with precipitation. These authors suggest that their different results compared to data published by other researchers can be due to several interacting genetic and environmental parameters. Additional strong correlations with precipitation and negatively with temperature suggest an inherently low drought tolerance of black currant plants. In our study, we have found that the level of total polyphenols in all parts of sweet cherry was higher in 2016 than 2015. It could be due to the short-term low temperature during ripening of sweet cherry fruit in May 2016. This observation was confirmed by Lee and Oh [41] who studied the impact of short-term low temperature on polyphenols content in kale. Other authors also detected this relationship, additionally showing an increase of anthocyanins [42].
Conclusion
The sweet cherry fruit, petioles and leaves are the source of bioactive compounds and show antioxidant activity. Statistically significant effect of cultivar, part of plant and weather conditions in individual harvest year on the chemical composition of sweet cherry was observed. There is a need for further research (especially in vivo study) of this material to find the potential benefits of sweet cherry fruit, petioles and leaves for the human health. This knowledge can be used in the prevention and treatment of the chronic non-communicable diseases. It brings new opportunities to create new functional food products and to better use the byproducts of sweet cherry production.
References
Stewart BW, Kleihues P (2003) World cancer report. IARC Press, Lyon. https://doi.org/10.1017/S0020860400079146
WHO (2012) Population-based approaches to childhood obesity prevention. WHO, Geneva
Potter JD, Finnegan JR, Guinard JX et al (2000) 5-a-day better health. Program evaluation report. National Institutes of Health, National Cancer Institute. NIH Publication No. 01-4904
Terry P, Giovannucci E, Michels KB et al (2001) Fruit, vegetables, dietary fiber, and risk of colorectal cancer. J Natl Cancer Inst 93:525–533. https://doi.org/10.1093/jnci/93.7.525
Ciok J, Szponar L (2006) Flawonoidy w diecie, a ryzyko powstawania i rozwoju chorob ukladu krazenia. Żywienie Człowieka i Metab 33:356–363
Korkina LG (2007) Phenylpropanoids as naturally occurring antioxidants: from plant defense to human health. Cell Mol Biol 53:15–25. https://doi.org/10.1170/T772
Ballistreri G, Continella A, Gentile A et al (2013) Fruit quality and bioactive compounds relevant to human health of sweet cherry (Prunus avium L.) cultivars grown in Italy. Food Chem 140:630–638. https://doi.org/10.1016/j.foodchem.2012.11.024
Kunachowicz H, Przygoda B, Nadolna I, Iwanow K (2017) Tabele składu i wartości odżywczej żywności [Food composition tables]. Wydawnictwo Lekarskie PZWL, Warsaw
Bastos C, Barros L, Dueñas M et al (2015) Chemical characterisation and bioactive properties of Prunus avium L.: the widely studied fruits and the unexplored stems. Food Chem 173:1045–1053. https://doi.org/10.1016/j.foodchem.2014.10.145
Giménez MJ, Valverde JM, Valero D et al (2016) Postharvest methyl salicylate treatments delay ripening and maintain quality attributes and antioxidant compounds of “Early Lory” sweet cherry. Postharvest Biol Technol 117:102–109. https://doi.org/10.1016/j.postharvbio.2016.02.006
Pacifico S, Di Maro A, Petriccione M et al (2014) Chemical composition, nutritional value and antioxidant properties of autochthonous Prunus avium cultivars from Campania Region. Food Res Int 64:188–199. https://doi.org/10.1016/j.foodres.2014.06.020
Ortega-García F, Blanco S, Peinado MA, Peragón J (2008) Polyphenol oxidase and its relationship with oleuropein concentration in fruits and leaves of olive (Olea europaea) cv. “Picual” trees during fruit ripening. Tree Physiol 28:45–54
Skupień K, Kostrzewa-Nowak D, Oszmiański J, Tarasiuk J (2008) In vitro antileukaemic activity of extracts from chokeberry (Aronia melanocarpa [Michx] Elliott) and mulberry (Morus alba L.) leaves against sensitive and multidrug resistant HL60 cells. Phyther Res 22:689–694. https://doi.org/10.1002/ptr.2411
Teleszko M, Wojdyło A (2015) Comparison of phenolic compounds and antioxidant potential between selected edible fruits and their leaves. J Funct Foods 14:736–746. https://doi.org/10.1016/j.jff.2015.02.041
Horwitz W, Latimer GW (2005) Official methods of analysis of AOAC International, 18th edn. AOAC International, Gaithersburg
Fortuna T, Rożnowski J (2012) Wybrane zagadnienia z chemii żywności: skrypt do ćwiczeń. Wydawnictwo Uniwersytetu Rolniczego, Kraków
Folch J, Lees M, Sloane Stanley GH (1957) A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226:497–509
Dziadek K, Kopeć A, Piątkowska E et al (2017) Identification of polyphenolic compounds and determination of antioxidant activity in extracts and infusions of buckwheat leaves. Eur Food Res Technol. https://doi.org/10.1007/s00217-017-2959-2
Benvenuti S, Pellati F, Melegari M, Bertelli D (2004) Polyphenols, anthocyanins, ascorbic acid, and radical scavenging activity of rubus, ribes, and aronia. Food Chem Toxicol 69:164–169
Goiffon JP, Mouly PP, Gaydolu E (1999) Anthocyanic pigment determination in the red fruit juices, concentrated juices and syrups using liquid chromatography. Anal Chem Acta 382:39–50
Re R, Pellegrini N, Proteggente A et al (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 26:1231–1237. https://doi.org/10.1016/S0891-5849(98)00315-3
Miliauskas G, Venskutonis PR, Van Beek TA (2004) Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem 85:231–237. https://doi.org/10.1016/j.foodchem.2003.05.007
Benzie I, Strain J (1996) The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem 239:70–76. https://doi.org/10.1006/abio.1996.0292
De Souza VR, Pereira PAP, Da Silva TLT et al (2014) Determination of the bioactive compounds, antioxidant activity and chemical composition of Brazilian blackberry, red raspberry, strawberry, blueberry and sweet cherry fruits. Food Chem 156:362–368. https://doi.org/10.1016/j.foodchem.2014.01.125
Rios JC, Robledo F, Schreiber L et al (2015) Association between the concentration of n-alkanes and tolerance to cracking in commercial varieties of sweet cherry fruits. Sci Hortic (Amsterdam) 197:57–65. https://doi.org/10.1016/j.scienta.2015.10.037
Schmitt ER, Feucht W (1993) Content of linolenic acid in senescing cherry leaves. Sci Hortic (Amsterdam) 55:273–282
Schmitz-Eiberger MA, Blanke MM (2012) Bioactive components in forced sweet cherry fruit (Prunus avium L.), antioxidative capacity and allergenic potential as dependent on cultivation under cover. LWT Food Sci Technol 46:388–392. https://doi.org/10.1016/j.lwt.2011.12.015
Usenik V, Stampar F, Petkovsek MM, Kastelec D (2015) The effect of fruit size and fruit colour on chemical composition in “Kordia” sweet cherry (Prunus avium L.). J Food Compos Anal 38:121–130. https://doi.org/10.1016/j.jfca.2014.10.007
Gonçalves B, Correia CM, Silva AP et al (2008) Leaf structure and function of sweet cherry tree (Prunus avium L.) cultivars with open and dense canopies. Sci Hortic (Amsterdam) 116:381–387. https://doi.org/10.1016/j.scienta.2008.02.013
Vagiri M, Ekholm A, Öberg E et al (2013) Phenols and ascorbic acid in black currants (Ribes nigrum L.): variation due to genotype, location, and year. J Agric Food Chem 61:9298–9306. https://doi.org/10.1021/jf402891s
Hayaloglu AA, Demir N (2015) Physicochemical characteristics, antioxidant activity, organic acid and sugar contents of 12 sweet cherry (Prunus Avium L.) cultivars grown in Turkey. J Food Sci 80:C564–C570. https://doi.org/10.1111/1750-3841.12781
Serradilla MJ, Lozano M, Bernalte MJ et al (2011) Physicochemical and bioactive properties evolution during ripening of “Ambrunés” sweet cherry cultivar. LWT Food Sci Technol 44:199–205. https://doi.org/10.1016/j.lwt.2010.05.036
Usenik V, Fabčič J, Štampar F (2008) Sugars, organic acids, phenolic composition and antioxidant activity of sweet cherry (Prunus avium L.). Food Chem 107:185–192. https://doi.org/10.1016/j.foodchem.2007.08.004
Prvulović D, Popović M, Malenčić Đ et al (2011) Phenolic compounds in sweet cherry (Prunus Avium L.) petioles and their antioxidant properties. Res J Agric Sci 43:198–202
Di Cagno R, Surico RF, Minervini G et al (2011) Exploitation of sweet cherry (Prunus avium L.) puree added of stem infusion through fermentation by selected autochthonous lactic acid bacteria. Food Microbiol 28:900–909. https://doi.org/10.1016/j.fm.2010.12.008
Serra AT, Duarte RO, Bronze MR, Duarte CMM (2011) Identification of bioactive response in traditional cherries from Portugal. Food Chem 125:318–325. https://doi.org/10.1016/j.foodchem.2010.07.088
Martini S, Conte A, Tagliazucchi D (2017) Phenolic compounds profile and antioxidant properties of six sweet cherry (Prunus avium) cultivars. Food Res Int 97:15–26. https://doi.org/10.1016/j.foodres.2017.03.030
Stagos D, Portesis N, Spanou C et al (2012) Correlation of total polyphenolic content with antioxidant and antibacterial activity of 24 extracts from Greek domestic Lamiaceae species. Food Chem Toxicol 50:4115–4124. https://doi.org/10.1016/j.fct.2012.08.033
Hakala M, Lapveteläinen A, Huopalahti R et al (2003) Effects of varieties and cultivation conditions on the composition of strawberries. J Food Compos Anal 16:67–80. https://doi.org/10.1016/S0889-1575(02)00165-5
Woznicki TL, Heide OM, Sønsteby A et al (2016) Effects of temperature and precipitation on yield and chemical composition of black currant fruits (Ribes nigrum L.). Acta Hortic. https://doi.org/10.17660/ActaHortic.2016.1133.27
Lee JH, Oh MM (2015) Short-term low temperature increases phenolic antioxidant levels in kale. Hortic Environ Biotechnol 56:588–596. https://doi.org/10.1007/s13580-015-0056-7
Caretto S, Linsalata V, Colella G et al (2015) Carbon fluxes between primary metabolism and phenolic pathway in plant tissues under stress. Int J Mol Sci 16:26378–26394. https://doi.org/10.3390/ijms161125967
Acknowledgements
The authors are grateful to Przemysław Banach for providing sweet cherry samples and Zbigniew Zuśka for sharing meteorological data. This study was financed by the National Science Centre, Poland (Grant no. 2015/17/N/NZ9/01136) as well as by the Ministry of Science and Higher Education of the Republic of Poland (Grant no. BM-4753/KŻCz/17).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Compliance with ethics requirements
This article does not contain any studies with human or animal subjects.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Dziadek, K., Kopeć, A. & Czaplicki, S. The petioles and leaves of sweet cherry (Prunus avium L.) as a potential source of natural bioactive compounds. Eur Food Res Technol 244, 1415–1426 (2018). https://doi.org/10.1007/s00217-018-3055-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-018-3055-y