Abstract
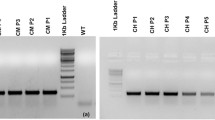
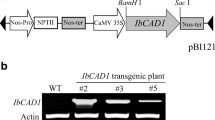
The impact of suppressing the caffeic acid O-methyltransferase (COMT) gene on lignin and fiber content as well as seed oil composition of Brassica napus was studied. Transgenic lines showed significant reduction in COMT enzyme activity ranging between 21 and 31 % residual activity. Lignin in the transgenic Cruciferin:COMT line 34-3 seeds was reduced by 35 % of the wild type, which decreased acid detergent fiber and neutral detergent fiber by 17.92 and 13.04 % of the control, respectively. The main fatty acids were monounsaturated (67.2–69.5 %) followed by polyunsaturated (20.9–23.3 %). The content of tocols ranged between 338 and 440 mg/100 g oil. The highest antiradical action toward 1,1-diphenyl-2-picrylhydrazyl and galvinoxyl radicals was in the oil of the transgenic line 34-3, which contained the highest amount of total phenolic compounds and total tocols. The results show the possibility of reducing lignin in B. napus with no effect on oil content and quality.





Similar content being viewed by others
Abbreviations
- COMT:
-
Caffeic acid O-methyltransferase
- DPPH:
-
1,1-Diphenyl-2-picrylhydrazyl radical
- NDF:
-
Neutral detergent fiber
- ADF:
-
Acid detergent fiber
- ADL:
-
Acid detergent lignin
- CTAB:
-
Cetyl trimethylammonium bromide
- FAME:
-
Fatty acid methyl esters
- HPLC:
-
High-performance liquid chromatography
- RSA:
-
Radical scavenging activity
References
Avramidou P, Evangelou A, Komilis D (2013) Use of municipal solid waste compost as a growth media for an energy plant (rapeseed). J Environ Manag 121:152–159
Pan M, Jiang T, Pan J (2011) Antioxidant activities of rapeseed protein hydrolysates. Food Bioprocess Technol 4:1144–1152
Bhinu V, Li R, Huang J, Kaminskyj S, Sharpe A, Hannoufa A (2009) Perturbation of lignin biosynthesis pathway in Brassica napus (canola) plants using RNAi. Can J Plant Sci 89:441–453
Statistics Canada (2012) Cereals and oil seed review-June 2012, Catalogue no. 22-007-X. Agriculture Division, Statistics Canada, Ottawa
Vanholme R, Demedts B, Morreel K, Ralph J, Boerjan W (2010) Lignin biosynthesis and structure. Plant Physiol 153:895–905
Junga H, Samaca D, Sarath G (2012) Modifying crops to increase cell wall digestibility. Plant Sci 185–186:65–77
Louie G, Bowman M, Tu Y, Mouradov A, Spangenberg G, Noel J (2010) Structure-function analyses of a caffeic acid O-methyltransferase from perennial ryegrass reveal the molecular basis for substrate preference. Plant Cell 22:4114–4127
Jung J, Vermerris W, Gallo M, Fedenko J, Erickson J, Altpeter F (2013) RNA interference suppression of lignin biosynthesis increases fermentable sugar yields for biofuel production from field-grown sugarcane. Plant Biotechnol J. doi:10.1111/pbi.12061
Meyer L, Gao J, Xu D, Thelen J (2012) Phosphoproteomic analysis of seed maturation in Arabidopsis, rapeseed, and soybean. Plant Physiol 59:517–528
Carpenter CD, Simon AE (1998) Preparation of RNA. In: Martinez-Zapater JM, Salinas J (eds) Methods in molecular biology: vol. 82, Arabidopsis protocols. Humana Press, Totowa, pp 85–89
Sambrook J, Fritsch E, Maniatis T (1989) Molecular cloning: a laboratory manual, vol 3. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Shewry P, Tatham A, Fido R (1995) Separation of plant proteins by electrophoresis. In: Jones H (ed) Methods in molecular biology—plant gene transfer and expression protocols, 49, 423–437. Humana Press, Totowa
Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Chen L, Auh C, Dowling P, Bell J, Lehmann D, Wang Z (2004) Transgenic down-regulation of caffeic acid O-methyltransferase (COMT) led to improved digestibility in tall fescue (Festuca arundinacea). Func Plant Biol 31:235–245
AOCS (1993) Official methods & recommended practices of the American Oil Chemists Society, 4th ed, edited by AOCS. Champaign, IL Official Method Ai 2 75, reapproved (2006)
ISO International Standard 5509 (2000) Animal and vegetable fats and oils-preparation of methyl esters of fatty acids. ISO, Geneva
Balz M, Shulte E, Their HP (1992) Trennung von tocopherol und tocotrienolen durch HPLC. Fat Sci Technol 94:209–213
Ramadan MF, Kinni SG, Seshagiri M, Mörsel JT (2010) Fat-soluble bioactives, fatty acid profile and radical scavenging activity of Semecarpus anacardium seed oil. J Amer Oil Chem Soc 87:885–894
Ramadan MF (2013) Healthy blends of high linoleic sunflower oil with selected cold pressed oils: functionality, stability and antioxidative characteristics. Ind Crops Prod 43:65–72
Casler M, Jung H (2006) Relationships of fibre, lignin, and phenolics to in vitro fibre digestibility in three perennial grasses. Anim Feed Sci Technol 125:151–161
Mertens D, McCaslin M (2008) Evaluation of alfalfa hays with down-regulated lignin biosynthesis. J Dairy Sci 91(Suppl. 1):170
Weakley D, Mertens D, McCaslin M (2008) Lactating cow responses to alfalfa hays with down-regulated lignin biosynthesis. J Dairy Sci 91(Suppl. 1):170
Wang Y, Ying J, Kuzma M (2005) Molecular tailoring of farnesylation for plant drought tolerance and yield protection. Plant J 43:413–424
Szydlowska-Czerniak A, Trokowski K, Karlovits G, Szlyk E (2010) Determination of antioxidant capacity, phenolic acids, and fatty acid composition of rapeseed varieties. J Agric Food Chem 58:7502–7509
Codex standard for named vegetable oils (Amended 2003, 2005). Codex standards for fats and oils from vegetable sources. CODEXSTAN 210; Codex Alimentarius Commission, FAO/WHO Food Standards Programme: Rome
Zanetti F, Vamerali T, Mosca G (2009) Yield and oil variability in modern varieties of high-erucic winter oilseed rape (Brassica napus L. var. oleifera) and Ethiopian mustard (Brassica carinata A. Braun) under reduced agricultural inputs. Ind Crops Prod 30:265–270
Marwede V, Mollers C, Olejniczak J, Becker HC (2003) Genetic variation, genotype-environment interactions and heritability of tocopherol content in winter oilseed rape (Brassica napus L.). In Proceedings of the 11th International Rapeseed Congress, KVL Copenhagen, 6–10, p 212–214
Koski A, Pekkarinen S, Hopia A, Wahala K, Heinonen M (2003) Processing of rapeseed oil: effects on sinapic acid derivative content and oxidative stability. Eur Food Res Technol 217:110–114
Koski A, Psomiadou E, Tsimidou M, Hopia A, Kefalas P, Wahala K, Heinonen M (2002) Oxidative stability and minor constituents of virgin olive oil and cold-pressed rapeseed oil. Eur Food Res Technol 214:294–298
Siger A, Nogala-Kalucka M, Lampart-Szczapa E (2008) The content and antioxidant activity if phenolic compounds in cold-pressed plant oils. J Food Lipids 15:137–149
Vuorela S, Meyer SA, Heinonen M (2004) Impact of isolation method on the antioxidant activity of rapeseed meal phenolics. J Agric Food Chem 52:8202–8207
Ramadan MF, Moersel J-T (2006) Screening of the antiradical action of vegetable oils. J Food Comp Anal 19:838–842
Acknowledgments
The authors gratefully acknowledge Dr. Abdelali Hannoufa, Southern Crop Protection and Food Research Centre, Agriculture and Agri-Food Canada, London, ON, Canada for guidance and providing the seeds. Also, authors would like to acknowledge Dr. Ahmad Omar, University of Florida, Gainesville, FL for his valuable contribution and constructive criticism during the manuscript preparation. This work was supported by Zagazig University, Zagazig, Egypt.
Conflict of interest
None.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oraby, H.F., Ramadan, M.F. Impact of suppressing the caffeic acid O-methyltransferase (COMT) gene on lignin, fiber, and seed oil composition in Brassica napus transgenic plants. Eur Food Res Technol 240, 931–938 (2015). https://doi.org/10.1007/s00217-014-2397-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-014-2397-3