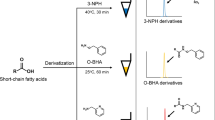
Abstract
Short-chain fatty acids (SCFAs) are the end products of the fermentation of complex carbohydrates by the gut microbiota. Although SCFAs are recognized as important markers to elucidate the link between gut health and disease, it has been difficult to analyze SCFAs with mass spectrometry technologies due to their poor ionization efficiency and high volatility. Here, we present a novel and sensitive method for the quantification of SCFAs, including C2–C6 SCFAs and their hydroxy derivatives, by liquid chromatography/tandem mass spectrometry (LC–MS/MS) upon N,N-dimethylethylenediamine (DMED) derivatization with a run time of 10 min. Moreover, the quantification method of DMED-derivatized SCFAs in intestinal contents using isotope-labeled internal standards was also established. The method validation was performed by analyzing spiked intestinal samples; the limits of detection and quantification of SCFAs with this method were found to be 0.5 and 5 fmol, respectively; the recovery was greater than 80% and good linearity (0.9932 to 0.9979) of calibration curves was obtained over the range from 0.005 to 5000 pmol/μL; the intraday and interday precisions were achieved in the range of 1–5%. Furthermore, the validated method was applied to analyze SCFAs in the cecum and colon contents of mice infected with the influenza virus. The results showed that the concentration of most of the SCFAs tested here decreased significantly in a time-dependent manner after the infection, suggesting a possibility that SCFAs in intestinal samples could be used as severe disease markers. Overall, we here successfully developed a simple, fast, and sensitive method for SCFA analysis by LC–MS/MS combined with DMED derivatization. The method for the quantification of SCFAs will be a useful tool for both basic research and clinical studies.
Graphical abstract






Similar content being viewed by others
References
Deleu S, Machiels K, Raes J, Verbeke K, Vermeire S (2021) Short chain fatty acids and its producing organisms: an overlooked therapy for IBD? eBioMedicine 2021;66:103293. https://doi.org/10.1016/J.EBIOM.2021.103293.
Cummings JH, Pomare EW, Branch HWJ, Naylor CPE, MacFarlane GT. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. 1987;28:1221–7. https://doi.org/10.1136/GUT.28.10.1221.
Kong D, Schipper L, van Dijk G. Distinct effects of short chain fatty acids on host energy balance and fuel homeostasis with focus on route of administration and host species. Front Neurosci. 2021;15:1434. https://doi.org/10.3389/FNINS.2021.755845/BIBTEX.
Nakkarach A, Foo HL, Song AAL, Mutalib NEA, Nitisinprasert S, Withayagiat U. Anti-cancer and anti-inflammatory effects elicited by short chain fatty acids produced by Escherichia coli isolated from healthy human gut microbiota. Microb Cell Fact. 2021;20:1–17. https://doi.org/10.1186/S12934-020-01477-Z/TABLES/3.
Li M, van Esch BCAM, Henricks PAJ, Folkerts G, Garssen J. The anti-inflammatory effects of short chain fatty acids on lipopolysaccharide- or tumor necrosis factor α-stimulated endothelial cells via activation of GPR41/43 and inhibition of HDACs. Front Pharmacol. 2018;9:533. https://doi.org/10.3389/FPHAR.2018.00533/BIBTEX.
Yao Y, Cai X, Fei W, Ye Y, Zhao M, Zheng C. The role of short-chain fatty acids in immunity, inflammation and metabolism. Crit Rev Food Sci Nutr. 2022;62:1–12. https://doi.org/10.1080/10408398.2020.1854675.
Canfora EE, Meex RCR, Venema K. Blaak EE (2019) Gut microbial metabolites in obesity, NAFLD and T2DM. Nat Rev Endocrinol. 2019;155(15):261–73. https://doi.org/10.1038/s41574-019-0156-z.
Zhang S, Wang H, Zhu MJ. A sensitive GC/MS detection method for analyzing microbial metabolites short chain fatty acids in fecal and serum samples. Talanta. 2019;196:249–54. https://doi.org/10.1016/j.talanta.2018.12.049.
Li M, Zhu R, Song X, Wang Z, Weng H, Liang J. A sensitive method for the quantification of short-chain fatty acids by benzyl chloroformate derivatization combined with GC-MS. Analyst. 2020;145:2692–700. https://doi.org/10.1039/D0AN00005A.
Wang HY, Wang C, Guo LX, Zheng YF, Hu WH, Dong TTX, Wang TJ, Tsim KWK. Simultaneous determination of short-chain fatty acids in human feces by HPLC with ultraviolet detection following chemical derivatization and solid-phase extraction segmental elution. J Sep Sci. 2019;42:2500–9. https://doi.org/10.1002/JSSC.201900249.
Cai J, Zhang J, Tian Y, Zhang L, Hatzakis E, Krausz KW, Smith PB, Gonzalez FJ, Patterson AD. Orthogonal comparison of GC-MS and 1H NMR spectroscopy for short chain fatty acid quantitation. Anal Chem. 2017;89:7900–6. https://doi.org/10.1021/ACS.ANALCHEM.7B00848/SUPPL_FILE/AC7B00848_SI_001.PDF.
Arellano M, Jomard P, El Kaddouri S, Roques C, Nepveu F, Couderc F. Routine analysis of short-chain fatty acids for anaerobic bacteria identification using capillary electrophoresis and indirect ultraviolet detection. J Chromatogr B Biomed Sci Appl. 2000;741:89–100. https://doi.org/10.1016/S0378-4347(00)00066-9.
Morrison DJ, Cooper K, Waldron S, Slater C, Weaver LT, Preston T. A streamlined approach to the analysis of volatile fatty acids and its application to the measurement of whole-body flux. Rapid Commun Mass Spectrom. 2004;18:2593–600. https://doi.org/10.1002/RCM.1662.
Shafaei A, Vamathevan V, Pandohee J, Lawler NG, Broadhurst D, Boyce MC. Sensitive and quantitative determination of short-chain fatty acids in human serum using liquid chromatography mass spectrometry. Anal Bioanal Chem. 2021;413:6333. https://doi.org/10.1007/S00216-021-03589-W.
Liebisch G, Ecker J, Roth S, Schweizer S, Öttl V, Schött HF, Yoon H, Haller D, Holler E, Burkhardt R, Matysik S (2019) Quantification of fecal short chain fatty acids by liquid chromatography tandem mass spectrometry—investigation of pre-analytic stability. Biomol 2019;9:121. https://doi.org/10.3390/BIOM9040121.
Zeng M, Cao H. Fast quantification of short chain fatty acids and ketone bodies by liquid chromatography-tandem mass spectrometry after facile derivatization coupled with liquid-liquid extraction. J Chromatogr B Analyt Technol Biomed Life Sci. 2018;1083:137–45. https://doi.org/10.1016/J.JCHROMB.2018.02.040.
Chan JCY, Kioh DYQ, Yap GC, Lee BW, Chan ECY. A novel LCMSMS method for quantitative measurement of short-chain fatty acids in human stool derivatized with 12 C- and 13 C-labelled aniline. J Pharm Biomed Anal. 2017;138:43–53. https://doi.org/10.1016/J.JPBA.2017.01.044.
Song HE, Lee HY, Kim SJ, Back SH, Yoo HJ. A facile profiling method of short chain fatty acids using liquid chromatography-mass spectrometry. Metabolites 2019;9(9):173. https://doi.org/10.3390/metabo9090173.
Xiong CF, Zhu QF, Chen YY, He DX, Feng YQ. Screening and identification of epoxy/dihydroxy-oxylipins by chemical labeling-assisted ultrahigh-performance liquid chromatography coupled with high-resolution mass spectrometry. Anal Chem. 2021;93:9904–11. https://doi.org/10.1021/ACS.ANALCHEM.1C02058.
Gowda SGB, Gowda D, Liang C, Li Y, Kawakami K, Fukiya S, Yokota A, Chiba H, Hui SP. Chemical labeling assisted detection and identification of short chain fatty acid esters of hydroxy fatty acid in rat colon and cecum contents. Metabolites. 2020;10(10):398. https://doi.org/10.3390/metabo10100398.
Zhu QF, Zhang Z, Liu P, Zheng SJ, Peng K, Deng QY, Zheng F, Yuan BF, Feng YQ. Analysis of liposoluble carboxylic acids metabolome in human serum by stable isotope labeling coupled with liquid chromatography-mass spectrometry. J Chromatogr A. 2016;1460:100–9. https://doi.org/10.1016/J.CHROMA.2016.07.017.
Gowda D, Ohno M, Siddabasave SG, Chiba H, Shingai M, Kida H. Hui SP (2021) Defining the kinetic effects of infection with influenza virus A/PR8/34 (H1N1) on sphingosine-1-phosphate signaling in mice by targeted LC/MS. Sci Reports. 2021;111(11):1–10. https://doi.org/10.1038/s41598-021-99765-0.
Siddabasave SG, Ikeda K, Arita M. Facile determination of sphingolipids under alkali condition using metal-free column by LC-MS/MS. Anal Bioanal Chem. 2018. https://doi.org/10.1007/s00216-018-1116-5.
Matuszewski BK, Constanzer ML, Chavez-Eng CM. Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC-MS/MS. Anal Chem. 2003;75:3019–30. https://doi.org/10.1021/AC020361S/ASSET/IMAGES/LARGE/AC020361SF00004.JPEG.
Hao YH, Zhang Z, Wang L, Liu C, Lei AW, Yuan BF, Feng YQ. Stable isotope labeling assisted liquid chromatography-electrospray tandem mass spectrometry for quantitative analysis of endogenous gibberellins. Talanta. 2015;144:341–8. https://doi.org/10.1016/J.TALANTA.2015.06.056.
Zhu QF, Yan JW, Zhang TY, Xiao HM, Feng YQ. Comprehensive screening and identification of fatty acid esters of hydroxy fatty acids in plant tissues by chemical isotope labeling-assisted liquid chromatography-mass spectrometry. Anal Chem. 2018;90(16):10056–63. https://doi.org/10.1021/acs.analchem.8b02839.
Zheng J, Zheng SJ, Cai WJ, Yu L, Yuan BF, Feng YQ. Stable isotope labeling combined with liquid chromatography-tandem mass spectrometry for comprehensive analysis of short-chain fatty acids. Anal Chim Acta. 2019;1070:51–9. https://doi.org/10.1016/J.ACA.2019.04.021.
Sencio V, Gallerand A, Machado MG, Deruyter L, Heumel S, Soulard D, Barthelemy J, Cuinat C, Vieira AT, Barthelemy A, Tavares LP, Guinamard R, Ivanov S, Grangette C, Teixeira MM, Foligné B, Wolowczuk I, Le Goffic R, Thomas M, Trottein F. Influenza virus infection impairs the gut’s barrier properties and favors secondary enteric bacterial infection through reduced production of short-chain fatty acids. Infect Immun 2021;89(9):e0073420. https://doi.org/10.1128/IAI.00734-20.
Sencio V, Barthelemy A, Tavares LP, Machado MG, Soulard D, Cuinat C, Queiroz-Junior CM, Noordine ML, Salomé-Desnoulez S, Deryuter L, Foligné B, Wahl C, Frisch B, Vieira AT, Paget C, Milligan G, Ulven T, Wolowczuk I, Faveeuw C, Le Goffic R, Thomas M, Ferreira S, Teixeira MM, Trottein F. Gut dysbiosis during influenza contributes to pulmonary pneumococcal superinfection through altered short-chain fatty acid production. Cell Rep. 2020;30:2934-2947.e6. https://doi.org/10.1016/j.celrep.2020.02.013.
Machado MG, Sencio V, Trottein F. Short-chain fatty acids as a potential treatment for infections: a closer look at the lungs. Infect Immun 2021; 89(9):e0018821. https://doi.org/10.1128/IAI.00188-21.
Haak BW, Littmann ER, Chaubard JL, Pickard AJ, Fontana E, Adhi F, Gyaltshen Y, Ling L, Morjaria SM, Peled JU, Van Den Brink MR, Geyer AI, Cross JR, Pamer EG, Taur Y. Impact of gut colonization with butyrate-producing microbiota on respiratory viral infection following allo-HCT. Blood. 2018;131:2978. https://doi.org/10.1182/BLOOD-2018-01-828996.
Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, Takahashi M, Fukuda NN, Murakami S, Miyauchi E, Hino S, Atarashi K, Onawa S, Fujimura Y, Lockett T, Clarke JM, Topping DL, Tomita M, Hori S, Ohara O, Morita T, Koseki H, Kikuchi J, Honda K, Hase K. Ohno H (2013) Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nat. 2013;5047480(504):446–50. https://doi.org/10.1038/nature12721.
Gowda SGB, Gowda D, Ohno M, Liang C, Chiba H, Hui S-P. Detection and structural characterization of SFAHFA homologous series in mouse colon contents by LTQ-Orbitrap-MS and their implication in influenza virus infection. J Am Soc Mass Spectrom. 2021;32:2196–205. https://doi.org/10.1021/JASMS.1C00138.
Funding
This work is supported by the Japanese Society for the Promotion of Science KAKENHI Grants (18K0743408, 19K0786109, 20K2335900, 21K1481201, and 21H02376) and by the Hokkaido Bureau of Economy, Trade, and Industry (strategic basic technology advancement support project).
Author information
Authors and Affiliations
Contributions
D.G., S.G.B.G.: conceptualization, methodology, supervision. Y.L., D.G., S.G.B.G.: formal analysis, visualization, writing—original draft. M.O., Y.L.: data curation, writing—review and editing. H.C., S.-P.H.: supervision, resources, writing—review and editing.
Corresponding author
Ethics declarations
Ethics approval
All the animal protocols were approved by the Animal Care and Use Committee of Hokkaido University (approval no. 17–0003).
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gowda, D., Li, Y., B Gowda, S. et al. Determination of short-chain fatty acids by N,N-dimethylethylenediamine derivatization combined with liquid chromatography/mass spectrometry and their implication in influenza virus infection. Anal Bioanal Chem 414, 6419–6430 (2022). https://doi.org/10.1007/s00216-022-04217-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-022-04217-x