Abstract
This work proposes a novel detection method for the ultra-sensitive colorimetric determination of lead and copper in complex water matrix. The method integrates signal amplification with analytical sensing, achieved by adsorptive preconcentration and a colorimetric assay. We report for the first time a strategic application of batch adsorption as a preconcentration method and colorimetry performed directly on the adsorbent surface enriched with metal. Commercially available kaolin was used as the adsorbent to preconcentrate the metals. The colorimetric detection of Pb and Cu was achieved using sodium rhodizonate and bathocuproine salt as chromogenic indicators, respectively. This method eliminates the involvement of complex instrumentation and the need for new sensing material preparation. The proposed method possesses high sensitivity for both Pb and Cu under optimized conditions. A linear calibration curve is obtained in two concentration ranges, spanning 1 to 100 µg L−1 with a low detection limit of 0.6 and 1.2 µg L−1 for Pb and Cu, respectively. Further, the method enables visual detection of Pb at concentrations as low as 2.5 µg L−1 by the naked eye. We demonstrate the practical applicability of the method by simultaneous detection of Pb and Cu in six different real-water samples with good apparent recovery % [90–120%]. Detection using hand-held devices indicates the feasibility for on-site analysis. Overall, this platform method offers a high scope for de-centralized monitoring of pollutants at concentrations which are prevailing in the environment.
Graphical abstract
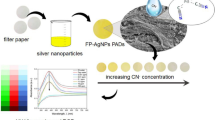
Integrating adsorptive preconcentration with colorimetric assay enables quantitative metal detection in environmental water sample matrix.







Similar content being viewed by others
References
UNICEF and WHO. 1 in 3 people globally do not have access to safe drinking water. Geneva: WHO News Release; 2019.
Briffa J, Sinagra E, Blundell R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon [Internet]. 2020;6(9):e04691. https://doi.org/10.1016/j.heliyon.2020.e04691.
Seiler C, Berendonk TU. Heavy metal driven co-selection of antibiotic resistance in soil and water bodies impacted by agriculture and aquaculture. Front Microbiol. 2012;3(DEC):1–10.
APHA. Standard methods for the examination of water and wastewater, vol. 02. American Public Health Association: American Water Works Association: Water Environment Federation; 2002. p. 1–541.
Ding R, Cheong YH, Ahamed A, Lisak G. Heavy metals detection with paper-based electrochemical sensors. Anal Chem. 2021;93(4):1880–8.
Liu J, Hu Q, Qi L, Lin JM, Yu L. Liquid crystal-based sensing platform for detection of Pb2+ assisted by DNAzyme and rolling circle amplification. J Hazard Mater. 2020;400(123218):1–6.
Sener G, Denizli A. Identification of several toxic metal ions using a colorimetric sensor array. In: Fitzgerald EJ, Fenniri H, editors. Biomimetic sensing: methods and protocols. Springer Nature; 2019. p. 81–6.
Dai C, Qian HL, Yan XP. Facile room temperature synthesis of ultra-small sized porous organic cages for fluorescent sensing of copper ion in aqueous solution. J Hazard Mater [Internet]. 2021;416(January):125860. https://doi.org/10.1016/j.jhazmat.2021.125860.
Dalmieda J, Kruse P. Metal cation detection in drinking water. Sensors (Switzerland). 2019;19(5134):1–44.
Yang Y, Noviana E, Nguyen MP, Geiss BJ, Dandy DS, Henry CS. Paper-based microfluidic devices: emerging themes and applications. Anal Chem. 2017;89(1):71–91.
Yan Z, Yuan H, Zhao Q, Xing L, Zheng X, Wang W, et al. Recent development of nanoenzyme-based colorimetric sensors for heavy metal detection and the interaction mechanism. Analyst. 2020;1106:115–25.
Liu B, Zhuang J, Wei G. Recent advances in the design of colorimetric sensors for environmental monitoring. Environ Sci Nano. 2020;7(8):2195–213.
Lace A, Cleary J. A review of microfluidic detection strategies for heavy metals in water. Chemosensors. 2021;9(60):1–26.
Yarur F, Macairan JR, Naccache R. Ratiometric detection of heavy metal ions using fluorescent carbon dots. Environ Sci Nano. 2019;6(4):1121–30.
Meredith NA, Quinn C, Cate DM, Reilly TH, Volckens J, Henry CS. Paper-based analytical devices for environmental analysis. Analyst. 2016;141(6):1874–87.
Nouri N, Khorram P, Duman O, Sibel T, Hassan S. Overview of nanosorbents used in solid phase extraction techniques for the monitoring of emerging organic contaminants in water and wastewater samples. Trends Environ Anal Chem. 2020;25(e0081):1–19.
Arena MP, Porter MD, Fritz JS. Rapid, low level determination of silver(I) in drinking water by colorimetric-solid-phase extraction. Anal Chim Acta. 2003;482(2):197–207.
Fritz JS, Arena MP, Steiner SA, Porter MD. Rapid determination of ions by combined solid-phase extraction-diffuse reflectance spectroscopy. J Chromatogr A. 2003;997(1–2):41–50.
Gazda DB, Fritz JS, Porter MD. Determination of nickel(II) as the nickel dimethylglyoxime complex using colorimetric solid phase extraction. Anal Chim Acta. 2004;508(1):53–9.
Gazda DB, Fritz JS, Porter MD. Multiplexed colorimetric solid-phase extraction: determination of silver(I), nickel(II), and sample pH. Anal Chem. 2004;76(16):4881–7.
Dias NC, Porter MD, Fritz JS. Principles and applications of colorimetric solid-phase extraction with negligible depletion. Anal Chim Acta. 2006;558(1–2):230–6.
Bradley MM, Siperko LM, Porter MD. Colorimetric-solid phase extraction method for trace level determination of arsenite in water. Talanta [Internet]. 2011;86(1):64–70. https://doi.org/10.1016/j.talanta.2011.08.004.
Feng L, Zhang Y, Wen L, Chen L, Shen Z, Guan Y. Colorimetric filtrations of metal chelate precipitations for the quantitative determination of nickel(II) and lead(II). Analyst. 2011;136(20):4197–203.
Feigl F, Suter HA. Analytical use of sodium rhodizonate. Ind Eng Chem - Anal Ed. 1942;14(10):840–2.
Bartsch MR, Kobus HJ, Wainwright KP. Update on the use of the sodium rhodizonate test for the detection of lead originating from firearm discharges. J Forensic Sci. 1996;41(6):1046–51.
Campos C, Guzman R, Lopez-Fernandez E, Casado A. Evaluation of the copper-II reduction assay using bathocuproinedisulfonic acid disodium salt for the total antioxidant capacity assessment: the CUPRAC-BCS assay. Anal Biochem. 2009;392:37–44.
Gu X, Evans LJ. Surface complexation modelling of Cd(II), Cu(II), Ni(II), Pb(II) and Zn(II) adsorption onto kaolinite. Geochim Cosmochim Acta. 2008;72(2):267–76.
Liu J, Sandaklie-Nikolova L, Wang X, Miller JD. Surface force measurements at kaolinite edge surfaces using atomic force microscopy. J Colloid Interface Sci [Internet]. 2014;420:35–40. https://doi.org/10.1016/j.jcis.2013.12.053.
Burns DT, Danzer K, Townshend A. Use of the terms “recovery” and “apparent recovery” in analytical procedures (IUPAC Recommendations 2002). Pure Appl Chem. 2002;74(11):2201–5.
Taverniers I, De Loose M, Van Bockstaele E. Trends in quality in the analytical laboratory. II. Analytical method validation and quality assurance. TrAC - Trends Anal Chem. 2004;23(8):535–52.
AOAC Official. AOAC guidelines for single laboratory validation of chemical methods for dietary supplements and botanicals. AOAC International; 2002.
Nguyen MN, Dultz S, Tran TTT, Bui ATK. Effect of anions on dispersion of a kaolinitic soil clay: a combined study of dynamic light scattering and test tube experiments. Geoderma [Internet]. 2013;209–210:209–13. https://doi.org/10.1016/j.geoderma.2013.06.024.
Adebowale KO, Unuabonah IE, Olu-Owolabi BI. Adsorption of some heavy metal ions on sulfate- and phosphate-modified kaolin. Appl Clay Sci. 2005;29(2):145–8.
Telliard WA. Determination of trace elements in water by preconcentration and inductively coupled plasma-mass spectrometry. U.S: Environmental Protection Agency; 1997.
Bernal E. Limit of detection and limit of quantification determination in gas chromatography. IntechOpen; 2014. p. 57–81.
Li JJ, Ji CH, Hou CJ, Huo DQ, Zhang SY, Luo XG, et al. High efficient adsorption and colorimetric detection of trace copper ions with a functional filter paper. Sens Actuat B Chem [Internet]. 2016;223:853–60. https://doi.org/10.1016/j.snb.2015.10.017.
Satarpai T, Shiowatana J, Siripinyanond A. Paper-based analytical device for sampling, on-site preconcentration and detection of ppb lead in water. Talanta [Internet]. 2016;154:504–10. https://doi.org/10.1016/j.talanta.2016.04.017.
Quinn CW, Cate DM, Miller-Lionberg DD, Reilly T, Volckens J, Henry CS. Solid-phase extraction coupled to a paper-based technique for trace copper detection in drinking water. Environ Sci Technol. 2018;52(6):3567–73.
Ministry of Health of The People’s Republic of China. Gb 5749–2006 Standards for Drinking Water Quality. 2006. p. 1–18.
Indian Standard Drinking Water Specification [Internet]. Vol. IS 10500, Bureau of Indian Standards. 2012. Available from: http://cgwb.gov.in/Documents/WQ-standards.pdf.
Canada H. Guidelines for Canadian Drinking Water Quality Summary Table Prepared by the Federal-Provincial-Territorial Committee on Drinking Water of the Federal-Provincial-Territorial Committee on Health and the Environment March 2006. Environments. 2012;2014:1–16.
DIRECTIVE (EU), on the quality of water intended for human consumption [Internet]. Vol. L 435, Official Journal of the European Communities. 2020. Available from: https://eur-lex.europa.eu/eli/dir/2020/2184/oj.
Acknowledgements
We gratefully acknowledge the financial support from the Department of Science and Technology, Government of India, National Environment Research Council (NERC), and Engineering and Physical Sciences Research Council (ESPRC) through the Indo-UK water quality research program (Grant: DST/TM/INDO-UK/2K17/46). The authors, R.S., M.M., and P.M., thank Rajesh Ghosh and Dr. G. Saranya from the Department of Chemical Engineering, IIT-Madras, for their assistance with the work.
Author information
Authors and Affiliations
Contributions
R.S. conceptualized and designed the experiments. R.S., P.M., and M.M. performed the experiments. R.S., T.R., and S.P. analyzed the results. T.R. and S.P. supervised the entire work and involved in funding acquisition. R.S. drafted original manuscript. T.R. and S.P. reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
216_2022_4056_MOESM1_ESM.docx
Supplementary file1 (DOCX 2374 KB) Data showing the effect of contact time for adsorptive preconcentration and metal detection; Effect of NaRh exposure to ambient air during Pb detection; Colour development in the adsorbent layer with respect to BCDS concentration for Cu detection, calibration saturation graphs, images without light illumination, and calibration graph.
Rights and permissions
About this article
Cite this article
Savitha, R., Mallelwar, P., Mohanraj, M. et al. Adsorptive preconcentration integrated with colorimetry for ultra-sensitive detection of lead and copper. Anal Bioanal Chem 414, 4089–4102 (2022). https://doi.org/10.1007/s00216-022-04056-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-022-04056-w