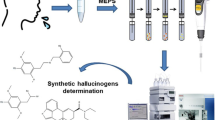
Abstract
This article deepens the potential of pre-cleaned bare wooden toothpicks (pb-WTs) for extracting drugs (antidepressants and acetaminophen) from oral fluid samples. The leaching of the intrinsic compounds from the wood matrix is identified as the main challenge for the final determination of the targets, even when a very selective instrumental technique, such as mass spectrometry, is employed. The pre-cleaning of the WTs is proposed for improving the analytical performance. The number of cleaning cycles depends on the injection mode (direct infusion or chromatography) into the mass spectrometer. The different variables affecting the extraction of selected antidepressant drugs were studied in detail, and the optimum procedure was validated using the two mentioned injection modes. The limits of detection were in the ranges 0.1–0.5 ng/mL and 0.1–0.3 ng/mL for direct infusion and liquid chromatography, respectively. The intra-day precision (expressed as relative standard deviation) was better than 12.1% and 8.6%, for direct infusion and liquid chromatography, respectively. Single-blind samples were used to study the applicability of the method. Finally, as a proof-of-concept, the potential of pb-WTs for in vivo sampling was outlined.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
White Analytical Chemistry (WAC) is a recently introduced concept which updates and complements the Green Analytical Chemistry (GAC) principles [1]. WAC establishes that new methods must balance sustainability with analytical usefulness. Although sample preparation is identified by GAC as a procedure with high potential environmental impact, it is accepted in the GAC-WAC context as it is essential to solving many analytical problems. Microextraction plays a pivotal role in sample preparation, and it involves the simplification and miniaturization of the techniques, thus reducing their environmental impact. As the tenth principle of GAC suggests, this impact can be further reduced by using reagents and materials obtained from renewable sources [2].
Natural materials are promising tools in microextraction due to their biodegradability, sustainability, and eco-friendliness character [3,4,5]. They are commonly modified to promote interaction with the target compounds. These modifications involve their superficial coating with polymers (e.g., polymeric ionic liquids (PILs) [6], molecularly imprinted polymers (MIPs) [7], commercial ones [8], nanoparticles [9], nanocomposites [10], and silanes [11]. Although the modification implies several advantages, bare biosorbents simplify and speed up the workflow and reduce the environmental impact related to their preparation. For this purpose, different unmodified biosorbents have been used in microextraction [12,13,14,15]. However, they require a previous cleaning to remove impurities or intrinsic components that may interfere in the instrumental determination of the target compounds [16, 17].
Wood emerges as an alternative sorbent for the extraction of analytes from complex samples. It is composed of cellulose, hemicellulose, lignin, and other minority intrinsic compounds. Due to its chemical composition, wood can establish hydrogen bonds and π-π interactions with target compounds of different polarities. Wooden toothpicks (WTs) are the most common extraction format, although wooden sticks [18] and wooden capillaries [19] have also been reported. Wooden-based sorbents also present rigidity and porosity. The versatility of WTs allows different sampling modalities including (i) sample deposition [20,21,22,23,24,25,26], (ii) dipping of liquid samples [27, 28], (iii) puncturing solid samples [29, 30], (iv) adhesion of granule/powdered samples [28, 31,32,33,34,35], and (v) conventional extraction-elution-analysis workflow [18, 36,37,38,39,40,41]. Moreover, the biocompatibility of wood derived from its lignocellulosic nature opens the door to in vivo sampling. In vivo sampling-extraction simplifies the analytical process minimizing the cross-contamination between samples and avoiding losses of chemical information during the sample storage/transportation [42,43,44]. An ideal in vivo technique should be affordable, cheap, and portable, with low solvent requirements, and should allow the isolation/preconcentration of the analytes [45].
The narrow shape of WTs permits their direct coupling to ambient ionization mass spectrometry (AIMS) in the so-called wooden-tip electrospray mass spectrometry (WT-ESI–MS) technique [46]. Although WT-ESI–MS is, by far, the most common technique when using WTs, direct infusion tandem mass spectrometry (DI-MS/MS) [41] and liquid chromatography-tandem mass spectrometry (LC–MS/MS) [18] have also been used.
In this article, pre-cleaned bare WTs (pb-WTs) have been successfully used to determine six tricyclic antidepressants (TCAs) in oral fluid samples by LC–MS/MS and DI-MS/MS. The effect of the pre-cleaning cycles, which only require boiled tap water, on the performance of both analytical techniques has been studied in-depth. The reduction of intrinsic interferents originating from the wood matrix decreases the ion suppression, enhancing the analytical sensitivity. The extraction workflow allows the simultaneous processing of multiple samples providing a sample throughput up to 6.5 and 12 samples/h for LC–MS/MS and DI-MS/MS, respectively. This aspect demonstrates the potential implementation of the method in routine laboratories. The proposed approach permits the analysis of human oral fluid samples as the linear range fits into the expected concentration of TCAs in this matrix. Single-blind spiked samples were used to demonstrate the applicability of the method. The potential of pb-WTs for in vivo sampling has been finally outlined using acetaminophen as a proof-of-concept analyte.
Materials and methods
Reagents
All the reagents were of analytical grade or better. Unless otherwise indicated, they were purchased from Sigma-Aldrich (Madrid, Spain). Stock standard solutions of the analytes (amitriptyline (AMI), clomipramine (CLO), desipramine (DES), imipramine (IMI), nortriptyline (NOR), and trimipramine (TRI)) were prepared at a concentration of 2000 mg/L in methanol (PanReac, Barcelona, Spain) and stored at – 20 °C. Acetaminophen was used as model analyte for in vivo sampling.
Clomipramine-d3 (CLO-d3), desipramine-d3 (DES-d3), and nortriptyline-d3 (NOR-d3) were used as internal standards (IS) in LC–MS/MS. IS stock standard solutions were prepared at a concentration of 100 mg/L in methanol and stored at – 20 °C. The signal of each analyte was corrected by its corresponding deuterated IS. The signal of TRI, IMI, and AMI was corrected using DES-d3 as no deuterated compounds were at our disposal.
Working solutions were prepared by diluting the stock solutions in methanol or Milli-Q water (Millipore Corp., Madrid, Spain) as required.
Methanol, Milli-Q water, and formic acid were used as components of the mobile phase in LC–MS/MS and DI-MS/MS analysis.
Bamboo WTs were purchased in a local market in Córdoba (Spain).
Oral fluid collection
The oral fluid blank samples used during the optimization were obtained using a Salivette® sample collection device (Sarstedt, Nümbrecht, Germany) following the procedure described in a previous publication of the group [41], with slight modifications. For clarity, the procedure is described in detail in the supplementary information. However, for the analysis of real samples, direct oral fluid spitting is recommended because of the retention ability of the cellulosic cotton roll towards the target analytes [47, 48]. Salivette® was used only during the optimization since it provides a large volume of samples more easily. Also, blank samples (obtained from a healthy volunteer who was not medicated with TCAs) were needed in the optimization/validation, so Salivette was fully compatible.
Preparation of the sorbents
Bamboo WTs (o.d. 2.3 mm) were cut in segments of 2.5 cm in length. The cut WTs were introduced into a 600-mL beaker with 400 mL of boiling water under magnetic agitation for 1 h. This process was repeated with fresh boiling water as demanded. Finally, the pb-WTs were dried at room temperature until their final use. Although other washing approaches (e.g., using overheated water or ultrasounds) are potentially applicable, the proposed approach is simple, affordable, and easy to develop in any laboratory.
Extraction procedure
The extraction procedure is depicted in Fig. 1. The pb-WTs were conditioned in Milli-Q water under magnetic agitation. Subsequently, pb-WTs were individually transferred into a glass vial containing 5 mL of the 1:4 diluted sample. The length of the sorbents allows for their entire immersion in the sample to maximize the practical surface area of the materials. The samples (up to 25, although the system allows the simultaneous extraction of 50) were simultaneously stirred in an orbital stirrer (Vibramax 110, Heidolph, Schwabach, Germany) for 1 h at 500 rpm. After the extraction step, each pb-WTs was washed with 2 mL of Milli-Q water in an Eppendorf tube (10 min, 500 rpm) to remove potential matrix interferences. The pb-WTs enriched with the analytes were dried with a tissue and transferred into an HPLC vial. The analytes were finally eluted with 200 μL of methanol for 30 min at 500 rpm. 5 µL of the eluate was analyzed by LC–MS/MS and DI-MS/MS for the identification and quantitation of the analytes.
The optimum pre-cleaning cycles depend on the final analysis strategy. Two cycles are used in LC–MS/MS, while 5 cycles are needed in DI-MS/MS.
LC–MS/MS and DI-MS/MS analysis
LC–MS/MS analyses were performed on an Agilent 1260 Infinity HPLC system (Agilent, Palo Alto, CA, USA) equipped with a binary high-pressure pump for mobile phase delivery and an autosampler. A guard column (0.2-μm filter, 2.1 mm) was employed to prevent the MS from potential particles. Chromatographic separation was carried out by reversed-phase using an Eclipse Plus C18 (4.6 × 100 mm, 3.5 μm) column. The mobile phase consisted of (A) aqueous solution with 0.1% (v/v) formic acid and (B) methanol, following an isocratic separation with a solvent ratio of 10:90 (v/v) for 7 min. DI-MS/MS analyses were performed in the absence of column using the same solvent ratio with a sample analysis of 1.5 min. The injection volume was 5 μL and the flow rate was maintained constant at 0.2 mL/min in both modalities. MS parameters are shown in Table S1.
Results and discussion
Effect of the WT pre-cleaning on the analytical signal
The use of bare lignocellulosic materials as sorbents is challenging since they present compounds that may leach during extraction or elution. These compounds can interfere not only with the extraction of the analytes, but also with their instrumental determination. In this sense, they are usually washed before use.
In the case of WTs, this situation is even more complicated as these materials have been mainly used in combination with direct MS analysis. The leaching of these intrinsic components hinders the efficient ionization of the analytes affecting the sensitivity of the method. This effect was evaluated in-depth in this article. For doing so, WTs were incubated with pure water following the procedure described in the supplementary information. After the incubation, the supernatant was spiked with the target analytes at 20 μg/L, and it was analyzed by DI-MS/MS. The results, including the signal provided by a standard prepared in Milli-Q at the same concentration for comparative purposes, are shown in Fig. S1. As can be observed, the presence of leached compounds from the wood matrix negatively affects to the ionization of the target compounds, thus resulting in a lower analytical signal. This point was evaluated in-depth considering the whole extraction procedure (isolation/elution) that it is described below.
The pre-cleaning of the WTs in water was considered an environmentally friendly strategy to avoid this shortcoming. For this purpose, the WTs were washed several times in boiling water, as reported in “Preparation of the sorbents,” and finally used to extract the analytes from aqueous standards. The eluates obtained in the extraction were analyzed by DI-MS/MS. As shown in Fig. 2A, the analytical signal for the analytes increased with the number of cleaning cycles. This aspect also benefits the spectrometer that is less exposed to dirty. Attending to the results, 5 pre-cleaning cycles were selected for DI-MS/MS.
The use of a chromatographic separation can also reduce ion suppression in MS. For this reason, the effect of the pre-cleaning cycles using LC–MS/MS as the instrumental technique was also evaluated. As is shown in Fig. 2B, the effect of the cycles on the signals is less pronounced when LC–MS/MS is used. In this case, 2 pre-cleaning cycles were selected as the optimum value. However, the instrument is more exposed in such conditions as the total number of co-eluted compounds is higher, although they do not influence the signal.
This reduction of the leaching effect opens the door to two different modalities: using pb-WTs with 2 washing cycles in LC–MS/MS or pb-WTs with 5 washing cycles in DI-MS/MS. The first approach implies longer analysis times (7 min/sample) but a more rapid preparation of the sorbent. The second approach implies faster sample analysis (1.5 min) but entails a longer sorbent preparation.
Effect of the different variables that affect the extraction
The variables that are involved in the extraction efficiency of the analytes were evaluated following a univariate approach using aqueous standards containing the TCAs and ISs at 20 ng/mL. In this case, the investigated variables were sample pH, ionic strength, and extraction time.
Firstly, the effect of the sample pH was studied in the interval from 3 to 10. More alkaline values were not evaluated to avoid the eventual damage of the WTs. The obtained results, expressed as absolute signal (peak area for each analyte), are shown in Fig. 3A. The extraction was favored at the physiological pH of the saliva (6–7) and, consequently, the pH adjustment of the samples is not mandatory.
The ionic strength can affect the extraction process in terms of kinetics (increasing viscosity diminish the mass transference) and thermodynamics (salting-out effect). The effect of the ionic strength was evaluated with the addition of NaCl to aqueous standards ranging from 0 to 1% (w/v). The conductance of the solutions was measured in a conductometer, and the resulting values ranged from 2.1 to 1.5·104 μmho/cm. Figure 3B represents the negative effect of the increasing salinity in the extraction of the analytes expressed as the absolute signal. However, the relative signal (corrected by the IS) is less affected by the ionic strength, making the adjustment not necessary.
As the matrix of the sample can also affect the extraction kinetics, the extraction time was studied using a pool of different blank saliva samples spiked with the analytes. The samples were diluted to minimize the negative effect of the viscosity in the extraction kinetics. A 1:4 dilution factor was selected according to our previous studies [41]. The studied interval spans from 5 to 60 min. Further extraction times were not evaluated to provide a high sample throughput. The extraction time had a positive effect on the extraction of the analytes both in aqueous standards (Fig. S2A) and spiked blank samples (Fig. S2B), and the equilibrium is not reached for 60 min. The extraction time was established at 60 min to guarantee the sensitivity of the method. This time does not affect the sample throughput too much because multiple samples can be extracted simultaneously.
Method validation
The analytical method was validated in terms of linearity, limit of detection (LOD), limit of quantification (LOQ), accuracy, and precision (inter and intra-day in both cases) using matrix-matched calibration curves for each analyte. The analytical method was validated, under optimized conditions, using diluted saliva with ultrapure Milli-Q water in a 1:4 dilution factor and fortified with known concentrations of each analyte. The validation was carried out by two different modalities: DI-MS/MS (Table 1) and LC–MS/MS (Table 2) using pb-WTs purified with 5 and 2 washing cycles, respectively. The linearity, with R2 > 0.9976, demonstrates an appropriate correlation. The linear range was calculated at seven different concentration levels ranging from 0.4 to 800 ng/mL, considering the dilution of the spiked samples. The LODs were calculated as 3 times the standard deviation of the intercept divided by the slope of the calibration graph. The LOQs were calculated as 10 times the standard deviation of the intercept divided by the slope of the calibration graph. Precision, determined as the relative standard deviation (RSD), was calculated to estimate the variation between different replicates. Intra-day precision and inter-day precision were calculated spiking the analytes at three different concentrations (4, 80, and 400 ng/mL), analyzing triplicates at three different days. Intra-day and inter-day precision, calculated at three different concentration levels, were lower than 8.6% and 14.3%, respectively, for LC–MS/MS, and lower than 9.3% and 16.4% for DI-MS/MS, respectively. Accuracy, expressed as relative recovery, was calculated on three different days, analyzing three levels of concentration (4, 80, and 400 ng/mL) by triplicate, varying between 77–121% and 94–133% for LC–MS/MS and DI-MS/MS, respectively.
Analysis of single-blind samples
The analysis of single-blind samples was carried out to evaluate the feasibility of the proposed method in analyzing samples with an unknown concentration of the analytes. These blind samples were prepared by the second author of the article and analyzed by the first author. In this case, three different spiked samples were analyzed by LC–MS/MS and DI-MS/MS, showing promising results in terms of qualitative and quantitative analyses (see Table 3). Both methodologies provided 100% of success in the identification of the compounds. Also, the quantitative analysis reported relative recoveries spanning from 69 to 89% for LC–MS/MS and from 69 to 85% for DI-MS/MS.
Conclusions and perspectives
Natural materials present great potential as sorbents in microextraction. Although they can be modified to boost the extraction capacity and/or selectivity, bare materials are environmentally friendly sorbents. In this article, pb-WTs have been evaluated as sorbents for isolating drugs from the oral fluid. The pre-cleaning of the WTs in water is essential to improve the analytical performance in MS under two injection modes (direct infusion and liquid chromatography). The direct infusion was more sensitive to co-eluted compounds from the wood matrix, requiring a larger number of pre-cleaning cycles. However, it provides a more rapid analysis compared to LC, also reducing the organic solvents required for each analysis.
The low price of WTs permits the cheap preparation of multiple extraction devices that can be disposed after being used. This aspect is also relevant from the bioanalytical point of view. It minimizes the exposure of the operators to contaminated devices and avoid cross-contamination as each sample is extracted with a new device.
WTs, especially if they are washed using pure water, are biocompatible thanks to their lignocellulosic nature. The potential use of this biocompatible material for in vivo sampling arose as a natural hypothesis during this project. This hypothesis has been outlined using acetaminophen as model analyte because positive samples for TCAs (ideal for this evaluation) were not finally available. Acetaminophen does not require a medical prescription, and it is used as a common pain reliever allowing the easy collection of positive samples . In this preliminary study, explained in detail in the supplementary information, pb-WTs provided promising results on extracting acetaminophen from the oral cavity allowing to follow the absorption profile of the drug (Fig. S3). This line will be further studied in future works.
References
Nowak PM, Wietecha-Posłuszny R, Pawliszyn J. White Analytical Chemistry: an approach to reconcile the principles of Green Analytical Chemistry and functionality. TrAC Trends Anal Chem. 2021;138: 116223. https://doi.org/10.1016/j.trac.2021.116223.
Gałuszka A, Migaszewski Z, Namieśnik J. The 12 principles of green analytical chemistry and the SIGNIFICANCE mnemonic of green analytical practices. TrAC Trends Anal Chem. 2013;50:78–84. https://doi.org/10.1016/j.trac.2013.04.010.
Mafra G, García-Valverde M, Millán-Santiago J, Carasek E, Lucena R, Cárdenas S. Returning to nature for the design of sorptive phases in solid-phase microextraction. Separations. 2019;7:2. https://doi.org/10.3390/separations7010002.
Pacheco-Fernández I, Allgaier-Díaz DW, Mastellone G, Cagliero C, Díaz DD, Pino V. Biopolymers in sorbent-based microextraction methods. TrAC Trends Anal Chem. 2020;125: 115839. https://doi.org/10.1016/j.trac.2020.115839.
Godage NH, Gionfriddo E. Use of natural sorbents as alternative and green extractive materials: a critical review. Anal Chim Acta. 2020;1125:187–200. https://doi.org/10.1016/j.aca.2020.05.045.
Ríos-Gómez J, García-Valverde MT, López-Lorente ÁI, Toledo-Neira C, Lucena R, Cárdenas S. Polymeric ionic liquid immobilized onto paper as sorptive phase in microextraction. Anal Chim Acta. 2020;1094:47–56. https://doi.org/10.1016/j.aca.2019.10.021.
Díaz-Liñán MC, López-Lorente AI, Cárdenas S, Lucena R. Molecularly imprinted paper-based analytical device obtained by a polymerization-free synthesis. Sensors Actuators B Chem. 2019;287:138–46. https://doi.org/10.1016/j.snb.2019.02.048.
Benedé JL, Chisvert A, Lucena R, Cárdenas S. Synergistic combination of polyamide-coated paper-based sorptive phase for the extraction of antibiotics in saliva. Anal Chim Acta. 2021;1164: 338512. https://doi.org/10.1016/j.aca.2021.338512.
Zhang J, Li Z, Zhang C, Feng B, Zhou Z, Bai Y, Liu H. Graphite-coated paper as substrate for high sensitivity analysis in ambient surface-assisted laser desorption/ionization mass spectrometry. Anal Chem. 2012;84:3296–301. https://doi.org/10.1021/ac300002g.
Casado-Carmona FA, Lucena R, Cárdenas S. Magnetic paper-based sorptive phase for enhanced mass transference in stir membrane environmental samplers. Talanta. 2021;228: 122217. https://doi.org/10.1016/j.talanta.2021.122217.
Saraji M, Farajmand B. Chemically modified cellulose paper as a thin film microextraction phase. J Chromatogr A. 2013;1314:24–30. https://doi.org/10.1016/j.chroma.2013.09.018.
Meng X, Liu Q, Ding Y. Paper-based solid-phase microextraction for analysis of 8-hydroxy-2’-deoxyguanosine in urine sample by CE-LIF. Electrophoresis. 2017;38:494–500. https://doi.org/10.1002/elps.201600439.
Díaz-Liñán MC, Lucena R, Cárdenas S, López-Lorente AI. Unmodified cellulose filter paper, a sustainable and affordable sorbent for the isolation of biogenic amines from beer samples. J Chromatogr A. 2021;1651: 462297. https://doi.org/10.1016/j.chroma.2021.462297.
Yao Y-N, Wu L, Di D, Yuan Z-C, Hu B. Vibrating tip spray ionization mass spectrometry for direct sample analysis. J Mass Spectrom. 2019;54:772–9. https://doi.org/10.1002/jms.4429.
Dias AN, Simão V, Merib J, Carasek E. Cork as a new (green) coating for solid-phase microextraction: determination of polycyclic aromatic hydrocarbons in water samples by gas chromatography–mass spectrometry. Anal Chim Acta. 2013;772:33–9. https://doi.org/10.1016/j.aca.2013.02.021.
Neves Dias A, Simão V, Merib J, Carasek E. Use of green coating (cork) in solid-phase microextraction for the determination of organochlorine pesticides in water by gas chromatography-electron capture detection. Talanta. 2015;134:409–14. https://doi.org/10.1016/j.talanta.2014.11.045.
Manzo V, Goya-Pacheco J, Arismendi D, Becerra-Herrera M, Castillo-Aguirre A, Castillo-Felices R, Rosero-Moreano M, Carasek E, Richter P. Cork sheet as a sorptive phase to extract hormones from water by rotating-disk sorptive extraction (RDSE). Anal Chim Acta. 2019;1087:1–10. https://doi.org/10.1016/j.aca.2019.08.069.
Karimiyan H, Hadjmohammadi MR, Kunjali KL, Moein MM, Dutta J, Abdel-Rehim M. Graphene oxide/polyethylene glycol-stick for thin film microextraction of β-blockers from human oral fluid by liquid chromatography-tandem mass Spectrometry. Molecules. 2019;24:3664. https://doi.org/10.3390/molecules24203664.
Lin J, Yan J, Xu Q, Wang X (2020) Study on properties of wooden capillary electrospray ionization mass spectrometry. Rapid Commun Mass Spectrom 34. https://doi.org/10.1002/rcm.8600
So P-K, Ng T-T, Wang H, Hu B, Yao Z-P. Rapid detection and quantitation of ketamine and norketamine in urine and oral fluid by wooden-tip electrospray ionization mass spectrometry. Analyst. 2013;138:2239. https://doi.org/10.1039/c3an36641c.
Yang B, Liu F, Wang L, Zou Y, Wang F, Deng W, Wan X, Yang X, He M, Huang O. Serum metabolic profiling study of endometriosis by using wooden-tip electrospray ionization mass spectrometry. Anal Methods. 2015;7:6125–32. https://doi.org/10.1039/C5AY01312G.
Yang B, Liu F, Guo J, Wan L, Wu J, Wang F, Liu H, Huang O. Rapid assay of neopterin and biopterin in urine by wooden-tip electrospray ionization mass spectrometry. Anal Methods. 2015;7:2913–6. https://doi.org/10.1039/C5AY00004A.
Hu B, Yao Z-P. Detection of native proteins using solid-substrate electrospray ionization mass spectrometry with nonpolar solvents. Anal Chim Acta. 2018;1004:51–7. https://doi.org/10.1016/j.aca.2017.11.079.
Ng T-T, So P-K, Hu B, Yao Z-P. Rapid detection and quantitation of drugs-of-abuse by wooden-tip electrospray ionization mass spectrometry. J Food Drug Anal. 2019;27:428–38. https://doi.org/10.1016/j.jfda.2018.09.002.
Wu L, YAO Y-N, YUAN Z-C, DI D, LI L, HU B,. Direct detection of lysozyme in viscous raw hen egg white binding to sodium dodecyl sulfonate by reactive wooden-tip electrospray ionization mass spectrometry. Anal Sci. 2020;36:341–6. https://doi.org/10.2116/analsci.19P288.
Ling C, Shi Q, Wei Z, Zhang J, Hu J, Pei J. Rapid analysis of quinones in complex matrices by derivatization-based wooden-tip electrospray ionization mass spectrometry. Talanta. 2022;237: 122912. https://doi.org/10.1016/j.talanta.2021.122912.
Yang Y, Deng J. Internal standard mass spectrum fingerprint: a novel strategy for rapid assessing the quality of Shuang-Huang-Lian oral liquid using wooden-tip electrospray ionization mass spectrometry. Anal Chim Acta. 2014;837:83–92. https://doi.org/10.1016/j.aca.2014.06.005.
Du Q, Deng J, Liu Y, Zhang X, Yang Y, Chen J. Rapid assessment of the quality of Qingkailing products using wooden-tip electrospray ionization mass spectrometry combined with multivariate statistical analysis. Anal Methods. 2015;7:4803–10. https://doi.org/10.1039/C5AY00448A.
Wu L, Yao Y, Hu B. Investigating distributions and changes of alkaloids in living <scp> Catharanthus roseus </scp> under low-phosphorus stress using wooden-tip electrospray ionisation mass spectrometry. Phytochem Anal. 2020;31:739–46. https://doi.org/10.1002/pca.2937.
Pereira HV, Pinto FG, dos Reis MR, Garrett TJ, Augusti R, Sena MM, Piccin E. A fast and effective approach for the discrimination of garlic origin using wooden-tip electrospray ionization mass spectrometry and multivariate classification. Talanta. 2021;230: 122304. https://doi.org/10.1016/j.talanta.2021.122304.
Yang Y, Deng J, Yao Z-P. Pharmaceutical analysis by solid-substrate electrospray ionization mass spectrometry with wooden tips. J Am Soc Mass Spectrom. 2014;25:37–47. https://doi.org/10.1007/s13361-013-0748-0.
Xin G-Z, Hu B, Shi Z-Q, Lam YC, Dong TT-X, Li P, Yao Z-P, Tsim KWK. Rapid identification of plant materials by wooden-tip electrospray ionization mass spectrometry and a strategy to differentiate the bulbs of Fritillaria. Anal Chim Acta. 2014;820:84–91. https://doi.org/10.1016/j.aca.2014.02.039.
Yang B, Wang F, Deng W, Zou Y, Liu F, Wan X, Yang X, Liu H, Huang O. Wooden-tip electrospray ionization mass spectrometry for trace analysis of toxic and hazardous compounds in food samples. Anal Methods. 2015;7:5886–90. https://doi.org/10.1039/C5AY00917K.
Yang Y, Deng J, Yao Z-P. Field-induced wooden-tip electrospray ionization mass spectrometry for high-throughput analysis of herbal medicines. Anal Chim Acta. 2015;887:127–37. https://doi.org/10.1016/j.aca.2015.06.025.
Hu B, Huang Y, Yin G, Zhang G, Zhang L, Wang T, Yao Z-P. Rapid detection of adulterated drugs in herbal dietary supplements by wooden-tip electrospray ionization mass spectrometry. Anal Methods. 2016;8:6840–6. https://doi.org/10.1039/C6AY01735E.
Deng J, Yang Y, Fang L, Lin L, Zhou H, Luan T. Coupling solid-phase microextraction with ambient mass spectrometry using surface coated wooden-tip probe for rapid analysis of ultra trace perfluorinated compounds in complex samples. Anal Chem. 2014;86:11159–66. https://doi.org/10.1021/ac5034177.
Huang Y, Ma Y, Hu H, Guo P, Miao L, Yang Y, Zhang M. Rapid and sensitive detection of trace malachite green and its metabolite in aquatic products using molecularly imprinted polymer-coated wooden-tip electrospray ionization mass spectrometry. RSC Adv. 2017;7:52091–100. https://doi.org/10.1039/C7RA10094A.
Deng J, Yu T, Yao Y, Peng Q, Luo L, Chen B, Wang X, Yang Y, Luan T. Surface-coated wooden-tip electrospray ionization mass spectrometry for determination of trace fluoroquinolone and macrolide antibiotics in water. Anal Chim Acta. 2017;954:52–9. https://doi.org/10.1016/j.aca.2016.12.008.
Hu B, So P-K, Yang Y, Deng J, Choi Y-C, Luan T, Yao Z-P. Surface-modified wooden-tip electrospray ionization mass spectrometry for enhanced detection of analytes in complex samples. Anal Chem. 2018;90:1759–66. https://doi.org/10.1021/acs.analchem.7b03675.
Liu Y, Yang Q, Chen X, Song Y, Wu Q, Yang Y, He L. Sensitive analysis of trace macrolide antibiotics in complex food samples by ambient mass spectrometry with molecularly imprinted polymer-coated wooden tips. Talanta. 2019;204:238–47. https://doi.org/10.1016/j.talanta.2019.05.102.
Millán-Santiago J, García-Valverde MT, Lucena R, Cárdenas S. Polyamide-coated wooden tips coupled to direct infusion mass spectrometry, a high throughput alternative for the determination of methadone, cocaine and methamphetamine in oral fluid. Microchem J. 2021;162: 105843. https://doi.org/10.1016/j.microc.2020.105843.
Bessonneau V, Boyaci E, Maciazek-Jurczyk M, Pawliszyn J. In vivo solid phase microextraction sampling of human saliva for non-invasive and on-site monitoring. Anal Chim Acta. 2015;856:35–45. https://doi.org/10.1016/j.aca.2014.11.029.
Wu L, Yuan Z-C, Li Z-M, Huang Z, Hu B. In vivo solid-phase microextraction swab sampling of environmental pollutants and drugs in human body for nano-electrospray ionization mass spectrometry analysis. Anal Chim Acta. 2020;1124:71–7. https://doi.org/10.1016/j.aca.2020.05.022.
Hu B, Ouyang G. In situ solid phase microextraction sampling of analytes from living human objects for mass spectrometry analysis. TrAC Trends Anal Chem. 2021;143: 116368. https://doi.org/10.1016/j.trac.2021.116368.
Musteata FM, Pawliszyn J. In vivo sampling with solid phase microextraction. J Biochem Biophys Methods. 2007;70:181–93. https://doi.org/10.1016/j.jbbm.2006.07.006.
Hu B, So P-K, Chen H, Yao Z-P. Electrospray ionization using wooden tips. Anal Chem. 2011;83:8201–7. https://doi.org/10.1021/ac2017713.
Vejar-Vivar C, García-Valverde MT, Mardones C, Lucena R, Cárdenas S. Polydopamine coated hypodermic needles as a microextraction device for the determination of tricyclic antidepressants in oral fluid by direct infusion MS/MS. RSC Adv. 2021;11:22683–90. https://doi.org/10.1039/D1RA02721B.
Sobczak Ł, Goryński K. Evaluation of swabs from 15 commercially available oral fluid sample collection devices for the analysis of commonly abused substances: doping agents and drugs of abuse. Analyst. 2020;145:7279–88. https://doi.org/10.1039/D0AN01379J.
Funding
The project PID2020-112862RB-I00 funded by MCIN/AEI/https://doi.org/10.13039/501100011033 (Feder “Una manera de hacer Europa) is gratefully acknowledged. J. Millán-Santiago expresses his gratitude for the predoctoral grant (FPU19/01488) from the Spanish Ministry of Science, Innovation, and Universities. This article is based upon work from the Sample Preparation Study Group and Network supported by the Division of Analytical Chemistry of the European Chemical Society.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Informed consent was obtained from all individual participants involved in the study. The study, which is in the framework of the PID2020-112862RB-I00 project, has been approved by the appropriate ethics committee (Comité de Ética de la Investigación de Córdoba) and has been performed in accordance with the ethical standards.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Published in the topical collection featuring Promising Early-Career (Bio)Analytical Researchers with guest editors Antje J. Baeumner, María C. Moreno-Bondi, Sabine Szunerits, and Qiuquan Wang.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Millán-Santiago, J., Lucena, R. & Cárdenas, S. Pre-cleaned bare wooden toothpicks for the determination of drugs in oral fluid by mass spectrometry. Anal Bioanal Chem 414, 5287–5296 (2022). https://doi.org/10.1007/s00216-022-03977-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-022-03977-w