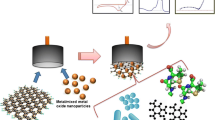
Abstract
This work presents the realization of a label-free electrochemical immunosensor for the quick, cheap, and straightforward determination of atrazine. This biodevice is based on developing a technological platform where a gold screen printed electrode (Au-SPE) surface was modified by the electrodeposition of a highly porous gold layer. As an internal probe redox, a Prussian Blue thin layer (PB) was then electrosynthetized onto the modified Au-SPE. Atrazine antibody (Ab-ATZ) was immobilized using G protein-functionalized magnetic nanoparticles (MNPs@protG) to ensure the correct orientation of the antibody to enhance the immunoaffinity. Under optimum experimental conditions, the electrochemical characterization of the developed immunosensor displays a linearity range towards atrazine within 0.05–1.5 ng/mL, a LOD of 0.011 ng/mL good reproducibility and stability. The immunosensor was tested in the analysis of spiked drinking water samples with a mean recovery ranging from 95.7 to 108.4%. The overall good analytical performances of this immunodevice suggest its application for the screening and monitoring of atrazine in real matrices.
Graphical Abstract










Similar content being viewed by others

References
Graymore M, Stagnitti F, Allinson G. Impacts of atrazine in aquatic ecosystems. Environ Int. 2001;26:483–95. https://doi.org/10.1016/S0160-4120(01)00031-9.
Ackerman F. The economics of atrazine. Int J Occup Environ Health. 2007;13:437–45. https://doi.org/10.1179/oeh.2007.13.4.437.
Friedmann AS. Atrazine inhibition of testosterone production in rat males following peripubertal exposure. Reprod Toxicol. 2002;16:275–9. https://doi.org/10.1016/S0890-6238(02)00019-9.
Samardzija D, Pogrmic-Majkic K, Fa S, Glisic B, Stanic B, Andric N. Atrazine blocks ovulation via suppression of Lhr and Cyp19a1 mRNA and estradiol secretion in immature gonadotropin-treated rats. Reprod Toxicol. 2016;61:10–8. https://doi.org/10.1016/j.reprotox.2016.02.009.
Abarikwu SO, Akiri OF, Durojaiye MA, Adenike A. Combined effects of repeated administration of Bretmont Wipeout (glyphosate) and Ultrazin (atrazine) on testosterone, oxidative stress and sperm quality of Wistar rats. Toxicol Mech Methods. 2015;25:70–80.
Hayes TB, Khoury V, Narayan A, Nazir M, Park A, Brown T, Adame L, Chan E, Buchholz D, Stueve T. Atrazine induces complete feminization and chemical castration in male African clawed frogs (Xenopus laevis). Proc Natl Acad Sci. 2010;107:4612–7.
Fraites MJP, Narotsky MG, Best DS, Stoker TE, Davis LK, Goldman JM, Hotchkiss MG, Klinefelter GR, Kamel A, Qian Y. Gestational atrazine exposure: effects on male reproductive development and metabolite distribution in the dam, fetus, and neonate. Reprod Toxicol. 2011;32:52–63.
Sánchez OF, Lin L, Bryan CJ, Xie J, Freeman JL, Yuan C. Profiling epigenetic changes in human cell line induced by atrazine exposure. Environ Pollut. 2020;258:1–11. https://doi.org/10.1016/j.envpol.2019.113712.
Sass JB, Colangelo A. European Union bans atrazine, while the United States negotiates continued use. Int J Occup Environ Health. 2006;12:260–7. https://doi.org/10.1179/oeh.2006.12.3.260.
IARC Monographs on the evaluation of carcinogenic risks to humans, WHO International Agency for Research on Cancer: Volume 73, IARCPress, 1999 Lyon, France
EPA US. Atrazine chemical summary toxicity and exposure assessment for children’s health. 2014;336–338 . https://doi.org/10.1016/B978-0-12-386454-3.00098-1
Lin C, Lerch RN, Garrett HE, George MF. Improved GC-MS/MS method for determination of atrazine and its chlorinated metabolites in forage plants—laboratory and field experiments. Commun Soil Sci Plant Anal. 2007;38:1753–73. https://doi.org/10.1080/00103620701435522.
Della-Flora A, Becker RW, Ferrão MF, Toci AT, Cordeiro GA, Boroski M, Sirtori C. Fast, cheap and easy routine quantification method for atrazine and its transformation products in water matrixes using a DLLME-GC/MS method. Anal Methods. 2018;10:5447–52. https://doi.org/10.1039/C8AY02227E.
Montiel-León JM, Vo Duy S, Munoz G, Bouchard MF, Amyot M, Sauvé S. Quality survey and spatiotemporal variations of atrazine and desethylatrazine in drinking water in Quebec, Canada. Sci Total Environ. 2019;671:578–85. https://doi.org/10.1016/j.scitotenv.2019.03.228.
Vanraes P, Willems G, Nikiforov A, Surmont P, Lynen F, Vandamme J, Van Durme J, Verheust YP, Van Hulle SWH, Dumoulin A, Leys C. Removal of atrazine in water by combination of activated carbon and dielectric barrier discharge. J Hazard Mater. 2015;299:647–55. https://doi.org/10.1016/j.jhazmat.2015.07.075.
Campuzano S, Yáñez-Sedeño P, Pingarrón JM. Electrochemical affinity biosensors based on selected nanostructures for food and environmental monitoring. Sensors (Switzerland). 2020;20:1–28. https://doi.org/10.3390/s20185125.
Lu LL, Suscovich TJ, Fortune SM, Alter G. Beyond binding: antibody effector functions in infectious diseases. Nat Rev Immunol. 2018;18:46–61. https://doi.org/10.1038/nri.2017.106.
Zumpano R, Polli F, D’Agostino C, Antiochia R, Favero G, Mazzei F. Nanostructure-based electrochemical immunosensors as diagnostic tools. Electrochem. 2021;2:10–28. https://doi.org/10.3390/electrochem2010002.
Zamfir LG, Geana I, Bourigua S, Rotariu L, Bala C, Errachid A, Jaffrezic-Renault N. Highly sensitive label-free immunosensor for ochratoxin A based on functionalized magnetic nanoparticles and EIS/SPR detection. Sensors Actuators, B Chem. 2011;159:178–84. https://doi.org/10.1016/j.snb.2011.06.069.
Song S, Kim YJ, Kang HL, Yoon S, Hong DK, Kim WH, Shin IS, Seong WK, Lee KN. Sensitivity Improvement in electrochemical immunoassays using antibody immobilized magnetic nanoparticles with a clean ITO working electrode. Biochip J. 2020;14:308–16. https://doi.org/10.1007/s13206-020-4309-x.
Matysiak-Brynda E, Bystrzejewski M, Wieckowska A, Grudzinski IP, Nowicka AM. Novel ultrasensitive immunosensor based on magnetic particles for direct detection of transferrin in blood. Sensors Actuators, B Chem. 2017;249:105–13. https://doi.org/10.1016/j.snb.2017.04.077.
Shen M, Rusling JF, Dixit CK. Site-selective orientated immobilization of antibodies and conjugates for immunodiagnostics development. Methods. 2017;116:95–111. https://doi.org/10.1016/j.ymeth.2016.11.010.
Akerstrom B, Brodin T, Reis K, Bjorck L. Protein G: a powerful tool for binding and detection of monoclonal and polyclonal antibodies. J Immunol. 1985;135(4):2589–92.
Gloag L, Mehdipour M, Chen D, Tilley RD, Gooding JJ. Advances in the application of magnetic nanoparticles for sensing. Adv Mater. 2019;31:1–26. https://doi.org/10.1002/adma.201904385.
Li Y, Song YY, Yang C, Xia XH. Hydrogen bubble dynamic template synthesis of porous gold for nonenzymatic electrochemical detection of glucose. Electrochem commun. 2007;9:981–8. https://doi.org/10.1016/j.elecom.2006.11.035.
Dawan S, Wannapob R, Kanatharana P, Limbut W, Numnuam A, Samanman S, Thavarungkul P. One-step porous gold fabricated electrode for electrochemical impedance spectroscopy immunosensor detection. Electrochim Acta. 2013;111:374–83. https://doi.org/10.1016/j.electacta.2013.08.012.
Chen X, Wang Y, Zhou J, Yan W, Li X, Zhu JJ. Electrochemical impedance immunosensor based on three-dimensionally ordered macroporous gold film. Anal Chem. 2008;80:2133–40. https://doi.org/10.1021/ac7021376.
Chen X, Zhou J, Xuan J, Yan W, Jiang LP, Zhu JJ. Room-temperature ionic liquid assisted fabrication of sensitive electrochemical immunosensor based on ordered macroporous gold film. Analyst. 2010;135:2629–36. https://doi.org/10.1039/c0an00264j.
Wang H, Zhao X, Yang H, Cao L, Deng W, Tan Y, Xie Q. Three-dimensional macroporous gold electrodes superior to conventional gold disk electrodes in the construction of an electrochemical immunobiosensor for: Staphylococcus aureus detection. Analyst. 2020;145:2988–94. https://doi.org/10.1039/c9an02392e.
Bollella P, Hibino Y, Kano K, Gorton L, Antiochia R. Highly sensitive membraneless fructose biosensor based on fructose dehydrogenase immobilized onto aryl thiol modified highly porous gold electrode: characterization and application in food samples. Anal Chem. 2018;90:12131–6. https://doi.org/10.1021/acs.analchem.8b03093.
Ricci F, Palleschi G. Sensor and biosensor preparation, optimisation and applications of Prussian Blue modified electrodes. Biosens Bioelectron. 2005;21:389–407. https://doi.org/10.1016/j.bios.2004.12.001.
Zhu P, Zhao Y. Cyclic voltammetry measurements of electroactive surface area of porous nickel: peak current and peak charge methods and diffusion layer effect. Mater Chem Phys. 2019;233:60–7. https://doi.org/10.1016/j.matchemphys.2019.05.034.
Trasatti S, Petrii OA. Real surface area measurements in electrochemistry. J Electroanal Chem. 1992;327:353–76. https://doi.org/10.1016/0926-860x(96)80148-7.
Bernalte E, Marín-Sánchez C, Pinilla-Gil E, Brett CMA. Characterisation of screen-printed gold and gold nanoparticle-modified carbon sensors by electrochemical impedance spectroscopy. J Electroanal Chem. 2013;709:70–6. https://doi.org/10.1016/j.jelechem.2013.09.007.
Butterworth A, Blues E, Williamson P, Cardona M, Gray L, Corrigan DK. SAM composition and electrode roughness affect performance of a DNA biosensor for antibiotic resistance. Biosensors. 2019;9.https://doi.org/10.3390/bios9010022
Burke LD, Nugent PF. The electrochemistry of gold: I. The redox behaviour of the metal in aqueous media. Gold Bull. 1997;30:43–53. https://doi.org/10.1007/BF03214756.
Yang J, Lin M, Cho MS, Lee Y. Determination of hydrogen peroxide using a Prussian Blue modified macroporous gold electrode. Microchim Acta. 2015;182:1089–94. https://doi.org/10.1007/s00604-014-1433-0.
Mokrushina AV, Heim M, Karyakina EE, Kuhn A, Karyakin AA. Enhanced hydrogen peroxide sensing based on Prussian Blue modified macroporous microelectrodes. Electrochem commun. 2013;29:78–80. https://doi.org/10.1016/j.elecom.2013.01.004.
Karyakin AA, Karyakina EE, Gorton L. On the mechanism of H2O2 reduction at Prussian Blue modified electrodes. Electrochem commun. 1999;1:78–82. https://doi.org/10.1016/S1388-2481(99)00010-7.
Ellis D, Eckhoff M, Neff VD. Electrochromism in the mixed-valence hexacyanides. 1. Voltammetric and spectral studies of the oxidation and reduction of thin films of Prussian blue. J Phys Chem. 1981;85:1225–31.
Itaya K, Ataka T, Toshima S. Spectroelectrochemistry and electrochemical preparation method of Prussian blue modified electrodes. J Am Chem Soc. 1982;104:4767–72. https://doi.org/10.1021/ja00382a006.
De Mattos IL, Gorton L, Ruzgas T, Karyakin AA. Sensor for hydrogen peroxide based on Prussian blue modified electrode: improvement of the operational stability. Anal Sci. 2000;16:795–8. https://doi.org/10.2116/analsci.16.795.
García-Jareño JJ, Sanmatías A, Vicente F, Gabrielli C, Keddam M, Perrot H. Study of Prussian blue (PB) films by ac-electrogravimetry: influence of PB morphology on ions movement. Electrochim Acta. 2000;45:3765–76. https://doi.org/10.1016/S0013-4686(00)00470-9.
Kulesza PJ, Zamponi S, Berrettoni M, Marassi R, Malik MA. Preparation, spectroscopic characterization and electrochemical charging of the sodium-containing analogue of Prussian blue. Electrochim Acta. 1995;40:681–8. https://doi.org/10.1016/0013-4686(94)00348-5.
Prabhu P, Babu RS, Narayanan SS. Synergetic effect of Prussian blue film with gold nanoparticle graphite-wax composite electrode for the enzyme-free ultrasensitive hydrogen peroxide sensor. J Solid State Electrochem. 2014;18:883–91. https://doi.org/10.1007/s10008-013-2288-8.
Ricci F, Palleschi G, Yigzaw Y, Gorton L, Ruzgas T. Investigation of the effect of different glassy carbon materials on the performance of Prussian blue based sensors for hydrogen peroxide. Electroanalysis. 2003;15(3):175–82. https://doi.org/10.1002/elan.200390021.
Liu X, Li WJ, Li L, Yang Y, Mao LG, Peng Z. A label-free electrochemical immunosensor based on gold nanoparticles for direct detection of atrazine. Sensors Actuators, B Chem. 2014;191:408–14. https://doi.org/10.1016/j.snb.2013.10.033.
Yang M, Zhao X, Zheng S, Liu X, Jin B, Li H, Gan Y. A new electrochemical platform for ultrasensitive detection of atrazine based on modified self-ordered Nb2O5 nanotube arrays. J Electroanal Chem. 2017;791:17–22. https://doi.org/10.1016/j.jelechem.2017.03.009.
Lavín Á, de Vicente J, Holgado M, Laguna MF, Casquel R, Santamaría B, et al. On the determination of uncertainty and limit of detection in label-free biosensors. Sensors (Switzerland). 2018;18.https://doi.org/10.3390/s18072038
Bhardwaj J, Devarakonda S, Kumar S, Jang J. Development of a paper-based electrochemical immunosensor using an antibody-single walled carbon nanotubes bio-conjugate modified electrode for label-free detection of foodborne pathogens. Sensors Actuators, B Chem. 2017;253:115–23. https://doi.org/10.1016/j.snb.2017.06.108.
Yılmaz E, Ozgur E, Bereli N, Turkmen D, Denizli A. Plastic antibody based surface plasmon resonance nanosensors for selective atrazine detection. Mater Sci Eng C. 2017;73:603–10. https://doi.org/10.1016/j.msec.2016.12.090.
Liu X, Yang Y, Mao L, Li Z, Zhou C, Liu X, Zheng S, Hu Y. SPR quantitative analysis of direct detection of atrazine traces on Au-nanoparticles: nanoparticles size effect. Sensors Actuators B Chem. 2015;218:1–7. https://doi.org/10.1016/j.snb.2015.04.099.
Marrakchi M, Helali S, Camino JS, González-Martínez MA, Abdelghani A, Hamdi M. Improvement of a pesticide immunosensor performance using site-directed antibody immobilisation and carbon nanotubes. Int J Nanotechnol. 2013;10:496–507. https://doi.org/10.1504/IJNT.2013.053519.
Rahbar N, Parham H. Carbon paste electrode modified with cuo-nanoparticles as a probe for square wave voltammetric determination of atrazine. Jundishapur J Nat Pharm Prod. 2013;8:118–24.
Belkhamssa N, Justino CIL, Santos PSM, Cardoso S, Lopes I, Duarte AC, Rocha-Santos T, Ksibi M. Label-free disposable immunosensor for detection of atrazine. Talanta. 2016;146:430–4. https://doi.org/10.1016/j.talanta.2015.09.015.
Shoorideh K, Chui CO. On the origin of enhanced sensitivity in nanoscale FET-based biosensors. Proc Natl Acad Sci U S A. 2014;111(14):5111–6. https://doi.org/10.1073/pnas.1315485111.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zumpano, R., Manghisi, M., Polli, F. et al. Label-free magnetic nanoparticles-based electrochemical immunosensor for atrazine detection. Anal Bioanal Chem 414, 2055–2064 (2022). https://doi.org/10.1007/s00216-021-03838-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-021-03838-y