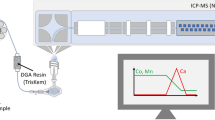
Abstract
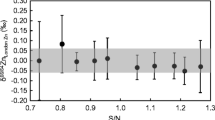
The application of Ca isotopic analysis in biomedical studies has great potential to identify changes in Ca metabolism and bone metabolism. Reliable measurement of Ca isotope-amount ratios is challenging considering limited Ca amounts and significant procedural blank levels. In this study, Ca purification was performed using the DGA Resin, optimized for low procedural blanks and separation of Ca from matrix elements and isobaric interferences (Na, Mg, K, Ti, Fe, Ba), while maintaining quasi-quantitative recoveries which are sufficient since a 42Ca–48Ca double-spike (DS) was applied. Ca isotopic analysis was performed using multicollector thermal ionization mass spectrometry (MC TIMS). The obtained procedural Ca blank of ≤10 ng enables processing of limited Ca amounts down to 670 ng. Data reduction of the measured Ca isotope-amount ratios was performed using an in-house developed software solving the DS algorithm. Data quality was improved by extension of equilibration time of the sample-DS mixture and implementation of a normalization strategy for raw isotopic data. The reported δ(44Ca/40Ca)NIST SRM 915a of NIST SRM 915a processed as a sample was found to be 0.01 ‰ ± 0.08 ‰ (2 SD, n = 15). Ca isotope-amount ratios of the reference material NIST SRM 1400 (bone ash), NIST SRM 1486 (bone meal), GBW07601 (human hair), and IAPSO (seawater) were in good agreement within uncertainty with literature data. Novel data on additional reference materials for biological tissues (hair) is presented, which might indicate a potential fractionation of Ca incorporated into hair tissue when compared to the blood pool.
Graphical abstract








Similar content being viewed by others
References
Morgan JLL, Skulan JL, Gordon GW, Romaniello SJ, Smith SM, Anbar AD. Rapidly assessing changes in bone mineral balance using natural stable calcium isotopes. Proc Natl Acad Sci. 2012;109(25):9989.
Eisenhauer A, Müller M, Heuser A, Kolevica A, Glüer CC, Both M, Laue C, Hehn U, Kloth S, Shroff R, Schrezenmeir J. Calcium isotope ratios in blood and urine: a new biomarker for the diagnosis of osteoporosis. Bone Rep. 2019;10:100200.
Heuser A, Frings-Meuthen P, Rittweger J, Galer SJG. Calcium isotopes in human urine as a diagnostic tool for bone loss: additional evidence for time delays in bone response to experimental bed rest. Front Physiol. 2019;10:12.
Tacail T, Le Houedec S, Skulan JL. New frontiers in calcium stable isotope geochemistry: perspectives in present and past vertebrate biology. Chem Geol. 2020;537:119471.
Chu N-C, Henderson GM, Belshaw NS, Hedges REM. Establishing the potential of ca isotopes as proxy for consumption of dairy products. Appl Geochem. 2006;21(10):1656–67.
Hirata T, Tanoshima M, Suga A, Tanaka Y, Nagata Y, Shinohara A, Chiba M. Isotopic analysis of calcium in blood plasma and bone from mouse samples by multiple collector-ICP-mass spectrometry. Anal Sci. 2008;24:1501–7.
Tacail T, Albalat E, Telouk P, Balter V. A simplified protocol for measurement of Ca isotopes in biological samples. J Anal At Spectrom. 2014;29:529–353.
Heuser A, Eisenhauer A, Scholz-Ahrens KE, Schrezenmeir J. Biological fractionation of stable Ca isotopes in Göttingen minipigs as a physiological model for Ca homeostasis in humans. Isot Environ Health Stud. 2016;52(6):633–48.
Tanaka Y-K, Mikuni-Takagaki Y, Hidaka K, Wada-Takahashi S, Kawamata R, Hirata T. Correction of mass spectrometric interferences for rapid and precise isotope ratio measurements of calcium from biological samples using ICP-mass spectrometry. Anal Sci. 2019;35(7):793–8.
Grigoryan R, Costas-Rodríguez M, Vandenbroucke RE, Vanhaecke F. High-precision isotopic analysis of Mg and Ca in biological samples using multi-collector ICP-mass spectrometry after their sequential chromatographic isolation – application to the characterization of the body distribution of Mg and Ca isotopes in mice. Anal Chim Acta. 2020;1130:137–45.
Skulan J, Bullen T, Anbar AD, Puzas JE, Shackelford L, LeBlanc A, Smith SM. Natural calcium isotopic composition of urine as a marker of bone mineral balance. Clin Chem. 2007;53(6):1155.
Heuser A, Eisenhauer A. A pilot study on the use of natural calcium isotope (44Ca/40Ca) fractionation in urine as a proxy for the human body calcium balance. Bone. 2010;46(4):889–96.
Morgan JLL, Gordon GW, Arrua RC, Skulan JL, Anbar AD, Bullen TD. High-precision measurement of variations in calcium isotope ratios in urine by multiple collector inductively coupled plasma mass spectrometry. Anal Chem. 2011;83(18):6956–62.
Channon MB, Gordon GW, Morgan JLL, Skulan JL, Smith SM, Anbar AD. Using natural, stable calcium isotopes of human blood to detect and monitor changes in bone mineral balance. Bone. 2015;77:69–74.
Rangarajan R, Mondal S, Thankachan P, Chakrabarti R, Kurpad AV. Assessing bone mineral changes in response to vitamin D supplementation using natural variability in stable isotopes of calcium in urine. Sci Rep. 2018;8(1):16751.
Lee S-H, Park S-J, Kim K-N, Cho D-Y, Kim Y-S, Kim B-T. Coronary calcification is reversely related with bone and hair calcium: the relationship among different calcium pools in body. J Bone Metab. 2016;23(4).
Gordon GW, Monge J, Channon MB, Wu Q, Skulan JL, Anbar AD, Fonseca R. Predicting multiple myeloma disease activity by analyzing natural calcium isotopic composition. Leukemia. 2014;28(10):2112–5.
Tanaka, Y-K, Yajima N, Higuchi Y, Yamato H, Hirata T. Calcium isotope signature: new proxy for net change in bone volume for chronic kidney disease and diabetic rats. Metallomics. 2017;9(12):1745–55.
Klevay LM, Bistrian BR, Fleming CR, Neumann CG. Hair analysis in clinical and experimental medicine. Am J Clin Nutr. 1987;46(2):233–6.
Bacsó J, Sarkadi L, Koltay E. On endogenous and exogenous calcium content of hair samples used in XRF and PIXE measurements. Int J Appl Radiat Isot. 1982;33(1):5–11.
MacPherson A, Bacsó J. Relationship of hair calcium concentration to incidence of coronary heart disease. Sci Total Environ. 2000;255(1):11–9.
Forte G, Alimonti A, Violante N, Di Gregorio M, Senofonte O, Petrucci F, Sancesario G, Bocca B. Calcium, copper, iron, magnesium, silicon and zinc content of hair in Parkinson’s disease. J Trace Elem Med Biol. 2005;19(2):195–201.
Miekeley N, de Carvalho Fortes LM, Porto da Silveira CL, Lima MB. Elemental anomalies in hair as indicators of endocrinologic pathologies and deficiencies in calcium and bone metabolism. J Trace Elem Med Biol 2001;15(1):46–55.
Park S-J, Lee S-H, Cho D-Y, Kim K-M, Lee D-J, Kim B-T. Hair calcium concentration is associated with calcium intake and bone mineral density. Int J Vitam Nutr Res. 2013;83(3):154–61.
Hinners TA, Terrill WJ, Kent JL, Colucci AV. Hair–metal binding. Environ Health Perspect. 1974;8:191–9.
Smart KE, Kilburn M, Schroeder M, Martin BGH, Hawes C, Marsh JM, Grovenor CRM. Copper and calcium uptake in colored hair. Int J Cosmet Sci. 2010;32(2):161–2.
Skulan J, DePaolo DJ. Calcium isotope fractionation between soft and mineralized tissues as a monitor of calcium use in vertebrates. Proc Natl Acad Sci. 1999;96(24):13709.
Rudge JF, Reynolds BC, Bourdon B. The double spike toolbox. Chem Geol. 2009;265(3–4):420–31.
Feng L, Zhou L, Yang L, Tong S, Hu Z, Gao S. Optimization of the double spike technique using peak jump collection by a Monte Carlo method: an example for the determination of Ca isotope ratios. J Anal At Spectrom. 2015;30(12):2403–11.
Fantle MS, Tipper ET. Calcium isotopes in the global biogeochemical Ca cycle: implications for development of a Ca isotope proxy. Earth-Sci Rev. 2014;129:148–77.
Wieser ME, Buhl D, Bouman C, Schwieters J. High precision calcium isotope ratio measurements using a magnetic sector multiple collector inductively coupled plasma mass spectrometer. J Anal At Spectrom. 2004;19(7):844.
Feng L, Zhou L, Yang L, Zhang W, Wang Q, Shuoyun T, Hu Z. A rapid and simple single-stage method for Ca separation from geological and biological samples for isotopic analysis by MC-ICP-MS. J Anal At Spectrom. 2018;33(3):413–21.
CIAAW. Calcium 2015 [Available from: https://www.ciaaw.org/calcium.htm].
Zhu HL, Zhang ZF, Wang GQ, Liu YF, Liu F, Li X, Sun WD. Calcium isotopic fractionation during ion-exchange column chemistry and thermal ionisation mass spectrometry (TIMS) determination. Geostand Geoanal Res. 2016;40(2):185–94.
Walczyk T. TIMS versus multicollector-ICP-MS: coexistence or struggle for survival? Anal Bioanal Chem. 2004;378(2):229–31.
Douthitt CB. The evolution and applications of multicollector ICPMS (MC-ICPMS). Anal Bioanal Chem. 2008;390(2):437–40.
Bürger S, Vogl J, Kloetzli U, Nunes L, Lavelle M. Chapter 14 Thermal ionisation mass spectrometry. Sector Field Mass Spectrometry for Elemental and Isotopic Analysis: The Royal Society of Chemistry; 2015. pp. 381–438.
Jakubowski N, Horsky M, Roos PH, Vanhaecke F, Prohaska T. Chapter 12 Inductively coupled plasma mass spectrometry. Sector Field Mass Spectrometry for Elemental and Isotopic Analysis: The Royal Society of Chemistry; 2015. pp. 208–318.
Chakrabarti R, Mondal S, Jacobson AD, Mills M, Romaniello SJ, Vollstaedt H. Review of techniques, challenges, and new developments for calcium isotope ratio measurements. Chem Geol. 2021;581:120398.
Reynard LM, Henderson GM, Hedges REM. Calcium isotope ratios in animal and human bone. Geochim Cosmochim Acta. 2010;74(13):3735–50.
Romaniello SJ, Field MP, Smith HB, Gordon GW, Kim MH, Anbar AD. Fully automated chromatographic purification of Sr and Ca for isotopic analysis. J Anal At Spectrom. 2015;30(9):1906–12.
Bao Z, Zong C, Chen K, Lv N, Yuan H. Chromatographic purification of Ca and Mg from biological and geological samples for isotope analysis by MC-ICP-MS. Int J Mass Spectrom. 2020;448:116268.
Skulan J, DePaolo DJ, Owens TL. Biological control of calcium isotopic abundances in the global calcium cycle. Geochim Cosmochim Acta. 1997;61(12):2505–10.
Clementz MT, Holden P, Koch PL. Are calcium isotopes a reliable monitor of trophic level in marine settings? Int J Osteoarchaeol. 2003;13(1–2):29–36.
Heuser A, Eisenhauer A. The calcium isotope composition (δ44/40Ca) of NIST SRM 915b and NIST SRM 1486. Geostand Geoanal Res. 2008;32(3):311–5.
Heuser A, Tütken T, Gussone N, Galer SJG. Calcium isotopes in fossil bones and teeth—diagenetic versus biogenic origin. Geochim Cosmochim Acta. 2011;75(12):3419–33.
Farkaš J, Déjeant A, Novák M, Jacobsen SB. Calcium isotope constraints on the uptake and sources of Ca2+ in a base-poor forest: a new concept of combining stable (δ44/42Ca) and radiogenic (εCa) signals. Geochim Cosmochim Acta. 2011;75(22):7031–46.
Huang S, Farkaš J, Yu G, Petaev MI, Jacobsen SB. Calcium isotopic ratios and rare earth element abundances in refractory inclusions from the Allende CV3 chondrite. Geochim Cosmochim Acta. 2012;77:252–65.
National Institute of Standards & Technology. Certificate of analysis - standard reference material 1400 bone ash. Gaithersburg: National Institute of Standards & Technology; 1992.
Driessens FCM, Verbeeck RK. Biominerals. Boca Raton: CRC Press; 1990.
Forte G, Bocca B, Senofonte O, Petrucci F, Brusa L, Stanzione P, Zannino S, Violante N, Alimonti A, Sancesario G. Trace and major elements in whole blood, serum, cerebrospinal fluid and urine of patients with Parkinson’s disease. J Neural Transmission. 2004;111(8):1031–40.
Harrington JM, Young DJ, Essader AS, Sumner SJ, Levine KE. Analysis of human serum and whole blood for mineral content by ICP-MS and ICP-OES: development of a mineralomics method. Biol Trace Elem Res. 2014;160(1):132–42.
Jeruszka-Bielak M, Brzozowska A. Relationship between nutritional habits and hair calcium levels in young women. Biol Trace Elem Res. 2011;144(1):63–76.
Mayer AJ, Wieser ME. The absolute isotopic composition and atomic weight of molybdenum in SRM 3134 using an isotopic double-spike. J Anal At Spectrom. 2014;29(1):85–94.
Yang L, Tong S, Zhou L, Hu Z, Mester Z, Meija J. A critical review on isotopic fractionation correction methods for accurate isotope amount ratio measurements by MC-ICP-MS. J Anal At Spectrom 2018.
Mohamed FAA. Identifying zinc inputs to Heard and McDonald Islands region using zinc concentrations and isotopic compositions. Calgary: University of Calgary; 2020.
Zimmermann T, Mohamed AF, Reese A, Wieser ME, Kleeberg U, Pröfrock D, Irrgeher J. Zinc isotopic variation of water and surface sediments from the German Elbe River. Sci Total Environ. 2020;707:135219.
Retzmann A, Zimmermann T, Pröfrock D, Prohaska T, Irrgeher J. A fully automated simultaneous single-stage separation of Sr, Pb, and Nd using DGA Resin for the isotopic analysis of marine sediments. Anal Bioanal Chem. 2017;409(23):5463–80.
Sasaki Y, Zhu Z-X, Sugo Y, Kimura T. Extraction of various metal ions from nitric acid to n-dodecanen by diglycolamide (DGA) compounds. J Nucl Sci Technol. 2007;44(3):405–9.
Pourmand A, Dauphas N. Distribution coefficients of 60 elements on TODGA resin: application to Ca, Lu, Hf, U and Th isotope geochemistry. Talanta. 2010;81:741–53.
Retzmann A, Walls D, Miller K, Wieser M, Irrgeher J, Prohask T. Assessing the potential of online ICP–MS analysis to optimize Ca/matrix separation using DGA Resin for subsequent isotopic analysis. Monatsh Chem. 2021;152:401–10.
Feng L-p, Zhou L, Yang L, DePaolo DJ, Tong S-Y, Liu Y-S, Owens TL, Gao S. Calcium isotopic compositions of sixteen USGS reference materials. Geostand Geoanal Res. 2017;41(1):93–106.
Kragten J. Tutorial review. Calculating standard deviations and confidence intervals with a universally applicable spreadsheet technique. Analyst. 1994;119(10):2161–5.
Amini M, Eisenhauer A, Böhm F, Holmden C, Kreissig K, Hauff F, Jochum KP. Calcium isotopes (δ44/40Ca) in MPI-DING reference glasses, USGS rock powders and various rocks: evidence for ca isotope fractionation in terrestrial silicates. Geostand Geoanal Res. 2009;33(2):231–47.
Huang S, Farkaš J, Jacobsen SB. Stable calcium isotopic compositions of Hawaiian shield lavas: evidence for recycling of ancient marine carbonates into the mantle. Geochim Cosmochim Acta. 2011;75(17):4987–97.
Steuber T, Buhl D. Calcium-isotope fractionation in selected modern and ancient marine carbonates. Geochim Cosmochim Acta. 2006;70(22):5507–21.
Hippler D, Witbaard R, van Aken HM, Buhl D, Immenhauser A. Exploring the calcium isotope signature of Arctica islandica as an environmental proxy using laboratory- and field-cultured specimens. Palaeogeogr Palaeoclimatol Palaeoecol. 2013;373:75–87.
Acknowledgements
The authors would like to thank Courtney Kruschel for her support in the lab and Alex Tennant for his support with the DS software.
Funding
This work was funded by the Chemical Monthly Fellowship (2018) of the Austrian Academy of Sciences (ÖAW), by the Discovery Research Grant awarded by the Natural Sciences and Engineering Research Council of Canada (NSERC), and by the Faculty of Science Grand Challenges Fund of the University of Calgary.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
No human participants or animals are involved in the present study. All analyzed biological tissue samples are commercially available certified reference materials.
Conflict of interest
The authors declare no competing interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Published in the topical collection celebrating ABCs 20th Anniversary.
Supplementary information
ESM 1
(DOCX 26 kb)
Rights and permissions
About this article
Cite this article
Retzmann, A., Walls, D., Miller, K.A. et al. A double-spike MC TIMS measurement procedure for low-amount Ca isotopic analysis of limited biological tissue samples. Anal Bioanal Chem 414, 675–689 (2022). https://doi.org/10.1007/s00216-021-03650-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-021-03650-8