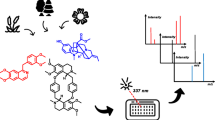
Abstract
Vulgarisins are members of diterpenoids with rare 5/6/4/5 ring skeleton from Prunella vulgaris Linn. (P. vulgaris). Their molecular scaffolds comprise different hydroxylation and degree of esterification. Vulgarisins have attracted many attentions in the fields of food and medicine for their potent bioactivities. Firstly, four reference compounds were analyzed by higher-energy collisional dissociation mass spectrometry (HCD MS/MS) and the fragmentation patterns for molecular scaffold were summarized. And then, a high-performance liquid chromatography/electrospray ionization/high-resolution mass spectrometry (HPLC-ESI-HR-MS) method was adopted to investigate the P. vulgaris extracts. Finally, the proposed analysis results were successfully applied to facilitate the discovery of the vulgarisins analogues from P. vulgaris. For the four reference compounds, the sodium adduct was the predominate ion in full scan. A specific fragmentation pathway of [M+Na]+ ions leads to produce diagnostic ions of vulgarisins at m/z 325 under HCD, which was formed through consecutive-side chains lost. Twenty-three diterpenoids, including 18 vulgarisins analogues, were identified or tentatively characterized in the botanical extracts of P. vulgaris based on their elemental constituents and characteristic fragment ion profiles. Two new vulgarisins analogues in the plant were isolated and their structures were illustrated based on extensive spectroscopic analysis using 1D and 2D nuclear magnetic resonance (NMR) spectroscopy. The HCD MS/MS method, including the profiles of the diagnostic ions induced by characteristic fragmentation, is an effective technique for the discovery of vulgarisins analogues in P. vulgaris. The expected fragmentation pattern knowledge will also facilitate the analysis of other natural products.
Graphical abstract







Similar content being viewed by others
References
Wang S-J, Wang X-H, Dai Y-Y, Ma M-H, Rahman K, Nian H, Zhang H. Prunella vulgaris: a comprehensive review of chemical constituents, pharmacological effects and clinical applications. Curr Pharm Design. 2019;25(3):359–69. https://doi.org/10.2174/1381612825666190313121608.
Lou H, Zheng S, Li T, Zhang J, Fei Y, Hao X, Liang G, Pan W. Vulgarisin A, a new diterpenoid with a rare 5/6/4/5 ring skeleton from the Chinese medicinal plant Prunella vulgaris. Org Lett. 2014;16(10):2696–9. https://doi.org/10.1021/ol5009763.
Qu Z, Zhang J, Yang H, Gao J, Chen H, Liu C, Gao W. Prunella vulgaris L., an edible and medicinal plant, attenuates scopolamine-induced memory impairment in rats. J Agric Food Chem. 2017;65(2):291–300. https://doi.org/10.1021/acs.jafc.6b04597.
Bai Y, Xia B, Xie W, Zhou Y, Xie J, Li H, Liao D, Lin L, Li C. Phytochemistry and pharmacological activities of the genus Prunella. Food Chem. 2016;204(8):483–96. https://doi.org/10.1016/j.foodchem.2016.02.047.
Lou H-Y, Jin L, Huang T, Wang D-P, Liang G-Y, Pan W-D. Vulgarisins B–D, three novel diterpenoids with a rare skeleton isolated from Prunella vulgaris Linn. Tetrahedron Lett. 2017;58(5):401–4. https://doi.org/10.1016/j.tetlet.2016.12.029.
Cai T, Guo Z-Q, Xu X-Y, Wu Z-J. Recent (2000–2015) developments in the analysis of minor unknown natural products based on characteristic fragment information using LC–MS. Mass Spectrom Rev. 2018;37(2):202–16. https://doi.org/10.1002/mas.21514.
Giles K, Ujma J, Wildgoose J, Pringle S, Richardson K, Langridge D, Green M. A cyclic ion mobility-mass spectrometry system. Anal Chem. 2019;91(13):8564–73. https://doi.org/10.1021/acs.analchem.9b01838.
Kim B, Araujo R, Howard M, Magni R, Liotta LA, Luchini A. Affinity enrichment for mass spectrometry: improving the yield of low abundance biomarkers. Expert Rev Proteomic. 2018;15(4):353–66. https://doi.org/10.1080/14789450.2018.1450631.
Johnson AR, Carlson EE. Collision-induced dissociation mass spectrometry: a powerful tool for natural product structure elucidation. Anal Chem. 2015;87(21):10668–78. https://doi.org/10.1021/acs.analchem.5b01543.
Vetere A, Alachraf MW, Panda SK, Andersson JT, Schrader W. Studying the fragmentation mechanism of selected components present in crude oil by collision-induced dissociation mass spectrometry. Rapid Commun Mass Sp. 2018;32(24):2141–51. https://doi.org/10.1002/rcm.8280.
Hsu F-F. Electrospray ionization with higher-energy collision dissociation tandem mass spectrometry toward characterization of ceramides as [M + Li]+ ions: mechanisms of fragmentation and structural identification. Anal Chim Acta. 2021;1142:221–34. https://doi.org/10.1016/j.aca.2020.09.056.
Ma F-W, Xia B, Luo S-H, Li S-H, Zhou Y. Analysis of the lithiated leucosceptroids from Leucosceptrum canum to facilitate their identification and differentiation by electrospray ionization tandem mass spectrometry. Rapid Commun Mass Sp. 2016;30(1):100–10. https://doi.org/10.1002/rcm.7632.
Zhang L, Ren ZY, Xia XP, Yang Q, Hong LB, Wu D. In situ determination of trace elements in melt inclusions using laser ablation inductively coupled plasma sector field mass spectrometry. Rapid Commun Mass Sp. 2019;33(4):361–70. https://doi.org/10.1002/rcm.8359.
Gómez Ramos MJ, Lozano A, Fernández-Alba AR. High-resolution mass spectrometry with data independent acquisition for the comprehensive non-targeted analysis of migrating chemicals coming from multilayer plastic packaging materials used for fruit purée and juice. Talanta. 2019;191:180–92. https://doi.org/10.1016/j.talanta.2018.08.023.
Getzinger GJ, Ferguson PL. Illuminating the exposome with high-resolution accurate-mass mass spectrometry and nontargeted analysis. Curr Opini Env Sci Health. 2020;15:49–56. https://doi.org/10.1016/j.coesh.2020.05.005.
Tsugawa H, Satoh A, Uchino H, Cajka T, Arita M, Arita M. Mass spectrometry data repository enhances novel metabolite discoveries with advances in computational metabolomics. Metabolites. 2019;9(6):119. https://doi.org/10.3390/metabo9060119.
Fu Q, Tong C, Guo Y, Xu J, Shi F, Shi S, Xiao Y. Flavonoid aglycone–oriented data-mining in high-performance liquid chromatography–quadrupole time-of-flight tandem mass spectrometry: efficient and targeted profiling of flavonoids in Scutellaria barbata. Anal Bioanal Chem. 2020;412(2):321–33. https://doi.org/10.1007/s00216-019-02238-7.
Rebryk A, Haglund P. Non-targeted screening workflows for gas chromatography–high-resolution mass spectrometry analysis and identification of biomagnifying contaminants in biota samples. Anal Bioanal Chem. 2020. https://doi.org/10.1007/s00216-020-03018-4.
Feng GF, Zheng Y, Sun Y, Liu S, Pi ZF, Song FR, Liu ZQ. A targeted strategy for analyzing untargeted mass spectral data to identify lanostane–type triterpene acids in Poria cocos by integrating a scientific information system and liquid chromatography–tandem mass spectrometry combined with ion mobility spectrometry. Anal Chim Acta. 2018;1033(11):87–99. https://doi.org/10.1016/j.aca.2018.06.048.
Silva R, Lopes NP, Silva DB. Application of MALDI mass spectrometry in natural products analysis. Planta Med. 2016;82(8):671–89. https://doi.org/10.1055/s-0042-104800.
Jágr M, Dvořáček V, Čepková PH, Doležalová J. Comprehensive analysis of oat avenanthramides using hybrid quadrupole-Orbitrap mass spectrometry: possible detection of new compounds. Rapid Commun Mass Sp. 2020;34(10):e8718. https://doi.org/10.1002/rcm.8718.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ma, F., Lou, H., Ge, Y. et al. Non-targeted analysis of vulgarisins by using collisional dissociation mass spectrometry for the discovery of analogues from Prunella vulgaris. Anal Bioanal Chem 413, 6513–6521 (2021). https://doi.org/10.1007/s00216-021-03615-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-021-03615-x