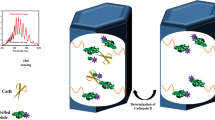
Abstract
A highly sensitive trypsin sensing system in serum was developed by using an anodic alumina oxide (AAO)-based, trypsin substrate-decorated hybrid ion permeation membrane. Owing to the trypsin-triggered peptide hydrolyzation reaction, the surface electrical feature of the peptide-decorated hybrid ion membrane changed. The electric double layer effect reduces the effective ion current diameter in the AAO nano unit, so that the ion current rectification ratio will be enhanced, realizing the quantitative detection of trypsin. The lowest detection concentration can be achieved as low as 0.1 pM. This method is no need for sample pre-preparation, easy to operate, highly sensitive, and also applicable to other enzyme evaluation systems by changing corresponding substrates. This study provides a new idea for selective measurements of proteases in complex biological samples.
Graphical abstract






Similar content being viewed by others
References
Huang S, Li F, Liao C, Zheng B, Du J, Xiao D. A selective and sensitive fluorescent probe for the determination of HSA and trypsin. Talanta. 2017. https://doi.org/10.1016/j.talanta.2017.01.034.
Zaccheo BA, Crooks RM. Self-powered sensor for naked-eye detection of serum trypsin. Anal Chem. 2011. https://doi.org/10.1021/ac103115z.
Yang L, Wu T, Fu C, Chen G, Xu S, Xu W. SERS determination of protease through a particle-on-a-film configuration constructed by electrostatic assembly in an enzymatic hydrolysis reaction. RSC Adv. 2016. https://doi.org/10.1039/C6RA15679G.
Zhang L, Du J. A sensitive and label-free trypsin colorimetric sensor with cytochrome c as a substrate. Biosens Bioelectron. 2016. https://doi.org/10.1016/j.bios.2015.12.070.
Hu JJ, Liu F, Ju HX. Peptide code-on-a-microplate for protease activity analysis via MALDI-TOF mass spectrometric quantitation. Anal Chem. 2015. https://doi.org/10.1021/acs.analchem.5b00230.
Seia MA, Stege PW, Pereira SV, De Vito IE, Raba J, Messina GA. Silica nanoparticle-based microfluidic immunosensor with laser-induced fluorescence detection for the quantification of immunoreactive trypsin. Anal Biochem. 2014. https://doi.org/10.1016/j.ab.2014.06.016.
Lefkowitz RB, Schmid-Schonbein GW, Heller MJ. Whole blood assay for trypsin activity using polyanionic focusing gel electrophoresis. Electrophoresis. 2010. https://doi.org/10.1002/elps.201000011.
Hu Q, Bao Y, Gan S, Zhang Y, Han D, Niu L. Electrochemically controlled grafting of polymers for ultrasensitive electrochemical assay of trypsin activity. Biosens Bioelectron. 2020. https://doi.org/10.1016/j.bios.2020.112358.
Chen Y, Lin Z, Miao C, Cai Q, Li F, Zheng Z, et al. A simple fluorescence assay for trypsin through a protamine-induced carbon quantum dot-quenching aggregation platform. RSC Adv. 2020. https://doi.org/10.1039/D0RA03970E.
Wuytens PC, Demol H, Turk N, Gevaert K, Skirtach AG, Lamkanfi M, et al. Gold nanodome SERS platform for label-free detection of protease activity. Faraday Discuss. 2017. https://doi.org/10.1039/c7fd00124j.
Wang H, Liu Q, Li W, Wen L, Zheng D, Bo Z, et al. Colloidal synthesis of lettuce-like copper sulfide for light-gating heterogeneous nanochannels. ACS Nano. 2016. https://doi.org/10.1021/acsnano.5b08079.
Han K, Heng L, Wen L, Jiang L. Biomimetic heterogeneous multiple ion channels: a honeycomb structure composite film generated by breath figures. Nanoscale. 2016. https://doi.org/10.1039/C6NR02506D.
Gao J, Guo W, Feng D, Wang H, Zhao D, Jiang L. High-performance ionic diode membrane for salinity gradient power generation. J Am Chem Soc. 2014. https://doi.org/10.1021/ja503692z.
Sun Z, Han C, Song M, Wen L, Tian D, Li H, et al. Fabrication of cysteine-responsive biomimetic single nanochannels by a thiolyne reaction strategy and their application for sensing in urine samples. Adv Mater. 2014. https://doi.org/10.1002/adma.201303158.
Siwy Z, Trofin L, Kohli P, Baker LA, Trautmann C, Martin CR. Protein biosensors based on biofunctionalized conical gold nanotubes. J Am Chem Soc. 2005. https://doi.org/10.1021/ja043910f.
Li SJ, Li J, Wang K, Wang C, Xu JJ, Chen HY, et al. A nanochannel array-based electrochemical device for quantitative label-free DNA analysis. ACS Nano. 2010. https://doi.org/10.1039/c7fd00124j.
Wang X, Smirnov S. Label-free DNA sensor based on surface charge modulated ionic conductance. ACS Nano. 2009. https://doi.org/10.1021/nn900113x.
Ma SS, Liu L, Xi GH, Ye Y, Wu LZ. The conductance change of nanoparticle translocation through silicon nitride nanopores. Nanosci Nanotechnol Lett. 2019. https://doi.org/10.1166/nnl.2019.2874.
Song J, Xu CH, Huang SZ, Lei W, Ruan YF, Lu HJ, et al. Ultrasmall panopipette: toward continuous monitoring of redox metabolism at subcellular level. Angew Chem Int Ed. 2018. https://doi.org/10.1002/anie.201808537.
Cao J, Zhao XP, Younis MR, Li ZQ, Xia XH, Wang C. Ultrasensitive capture, detection, and release of circulating tumor cells using a nanochannel ion channel hybrid coupled with electrochemical detection technique. Anal Chem. 2017. https://doi.org/10.1021/acs.analchem.7b02765.
Su T, He L, Mo R, Zhou C, Wang Z, Wang Y, et al. A non-enzymatic uric acid sensor utilizing ion channels in the barrier layer of a porous anodic alumina membrane. Electrochem Commun. 2018. https://doi.org/10.1016/j.elecom.2018.10.017.
Zhao XP, Liu FF, Hu WC, Younis MR, Wang C, Xia X-H. Biomimetic nanochannel-ionchannel hybrid for ultrasensitive and label-free detection of microRNA in cells. Anal Chem. 2019. https://doi.org/10.1021/acs.analchem.8b05536.
Zhao XP, Zhou Y, Zhang QW, Yang DR, Wang C, Xia XH. Nanochannel-ion channel hybrid device for ultrasensitive monitoring of biomolecular recognition events. Anal Chem. 2018. https://doi.org/10.1021/acs.analchem.8b05162.
Masuda H, Fukuda K. Ordered metal nanohole arrays made by a two-step replication of honeycomb structures of anodic alumina. Science. 1995. https://doi.org/10.1126/science.268.5216.1466.
Wang C, Zhao XP, Liu FF, Chen Y, Xia XH, Li J. Dendrimer-Au nanoparticle network covered alumina membrane for ion rectification and enhanced bioanalysis. Nano Lett. 2020. https://doi.org/10.1021/acs.nanolett.9b05066.
Kuo CH, Chang HY, Liu C-P, Lee SH, You YW, Shyue JJ. Effect of surface chemical composition on the surface potential and iso-electric point of silicon substrates modified with self-assembled monolayers. Phys Chem Chem Phys. 2011. https://doi.org/10.1039/c0cp02615h.
Feng Z, Liu Q, Zhang H, Xu D, Zhai H, Ren H. Adsorption of bovine serum albumin on the surfaces of poplar lignophenols. Int J Biol Macromol. 2020. https://doi.org/10.1016/j.ijbiomac.2020.04.270.
Kong W, Li Q, Xia L, Li X, Sun H, Kong RM, et al. Photoelectrochemical determination of trypsin by using an indium tin oxide electrode modified with a composite prepared from MoS2 nanosheets and TiO2 nanorods. Microchim Acta. 2019. https://doi.org/10.1007/s00604-019-3589-0.
Duan X, Li N, Wang G, Su X. High sensitive ratiometric fluorescence analysis of trypsin and dithiothreitol based on WS2 QDs. Talanta. 2020. https://doi.org/10.1016/j.talanta.2020.121171.
Funding
This work was supported by the National Natural Science Foundation of China (21873039), the Project was Supported by the State Key Laboratory of Applied Optics (sklssm2021016), and Interdisciplinary Integration Innovation Project of Jilin University (JLUXKJC2020106).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethics approval
For ethical reasons, we need to stress that the serum used in the experiment was bovine serum. We did not use human serum in the study.
Consent to participate
Informed consent was given by all study participants.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 992 kb)
Rights and permissions
About this article
Cite this article
Guan, S., Yue, J., Sun, W. et al. Ultrasensitive detection of trypsin in serum via nanochannel device. Anal Bioanal Chem 413, 4939–4945 (2021). https://doi.org/10.1007/s00216-021-03491-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-021-03491-5