Abstract
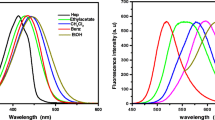
We present a facile method for the preparation of red-emitting and water-soluble silver nanoclusters (Ag NCs) using dihydrolipoic acid and sodium borohydride as the template and reducing agent. Ethanol solvent is demonstrated to endow Ag NCs with dramatically enhanced fluorescence; therefore, the Ag NCs are synthesized in ethanol/water solution (e/w-Ag NCs) instead of aqueous solution. Specific trivalent chromium (Cr3+) recognition capability of the e/w-Ag NCs can thus be obtained on the basis of its fluorescence quenching. The mechanisms for fluorescence enhancement and quenching of the e/w-Ag NCs triggered by ethanol and Cr3+, respectively, are investigated in detail. Next, a fluorescence method for detection of Cr3+ is established and its analytical performance is evaluated: the detection limit for Cr3+ is 0.71 μM and the linear range is from 2 to 40 μM. The fluorescent probe exhibits sufficient sensitivity and good selectivity toward Cr3+, illustrating that it has great promise for practical application in Cr3+ detection.






Similar content being viewed by others
References
Hassanien MM, Kenawy IM, El-Menshawy AM, El-Asmy AA. A novel method for speciation of Cr(III) and Cr(VI) and individual determination using Duolite C20 modified with active hydrazine. J Hazard Mater. 2008;158:170–6.
Latmani RB, Obraztsova A, Mackey MR, Ellisman MH, Tebo BM. Toxicity of Cr(III) to Shewanella sp. strain MR-4 during Cr(VI) reduction. Environ Sci Technol. 2007;41:214–20.
Eastmond DA, MacGregor JT, Slesinski RS. Trivalent chromium: assessing the genotoxic risk of an essential trace element and widely used human and animal nutritional supplement. Crit Rev Toxicol. 2008;38:173–90.
Zhou Y, Zhang J, Zhang L, Zhang Q, Ma T, Niu J. A rhodamine-based fluorescent enhancement chemosensor for the detection of Cr3+ in aqueous media. Dyes and Pigments. 2013;97:148–54.
Peralta-Videa JR, Lopez ML, Narayan M, Saupe G, Gardea-Torresdey J. The biochemistry of environmental heavy metal uptake by plants: implications for the food chain. Int J Biochem Cell B. 2009;41:1665–77.
Cathum S, Brown CE, Wong W. Determination of Cr3+,CrO4 2–, and Cr2O7 2– in environmental matrixes by high-performance liquid chromatography with diode-array detection (HPLC–DAD). Anal Bioanal Chem. 2002;373:103–10.
Ganjali MR, Emami M, Salavati-Niasari M, Yousefi M. Determination of trace amounts of Cr(III) in presence of Cr(VI) by a novel potentiometric membrane sensor based on a new tridentate S,N,O Schiff's base. Anal Lett. 2003;36:2735–47.
Tunçeli A, Türker AR. Speciation of Cr(III) and Cr(VI) in water after preconcentration of its 1,5-diphenylcarbazone complex on amberlite XAD-16 resin and determination by FAAS. Talanta. 2002;57:1199–204.
Kaewkhomdee N, Mounicou S, Szpunar J, Lobinski R, Shiowatana J. Characterization of binding and bioaccessibility of Cr in Cr-enriched yeast by sequential extraction followed by two-dimensional liquid chromatography with mass spectrometric detection. Anal Bioanal Chem. 2010;396:1355–64.
Shellaiah M, Simon T, Sun KW, Ko FH. Simple bare gold nanoparticles for rapid colorimetric detection of Cr3+ ions in aqueous medium with real sample applications. Sens Actuators B. 2016;226:44–51.
Boonmee C, Noipa T, Tuntulani T, Ngeontae W. Cysteamine capped CdS quantum dots as a fluorescence sensor for the determination of copper ion exploiting fluorescence enhancement and long-wave spectral shifts. Spectrochim Acta A. 2016;169:161–8.
Wu YS, Li CY, Li YH, Tang JL, Liu D. A ratiometric fluorescent chemosensor for Cr3+ based on monomer–excimer conversion of a pyrene compound. Sens Actuators B. 2014;203:712–8.
Wu S, Zhang K, Wang Y, Mao D, Liu X, Yu J, et al. A novel Cr3+ turn-on probe based on naphthalimide and BINOL framework. Tetrahedron Lett. 2014;55:351–3.
Liu S, Lu F, Zhu JJ. Highly fluorescent Ag nanoclusters: microwave-assisted green synthesis and Cr3+ sensing. Chem Commun. 2011;47:2661–3.
Zhang L, Wang E. Metal nanoclusters: New fluorescent probes for sensors and bioimaging. Nano Today. 2014;9:132–57.
Zhang N, Si Y, Sun Z, Chen L, Li R, Qiao Y, et al. Rapid, selective, and ultrasensitive fluorimetric analysis of mercury and copper levels in blood using bimetallic gold–silver nanoclusters with “silver effect”-enhanced red fluorescence. Anal Chem. 2014;86:11714–21.
Feng L, Liu M, Liu H, Fan C, Cai Y, Chen L, et al. High-throughput and sensitive fluorimetric strategy for microRNAs in blood using wettable microwells array and silver nanoclusters with red fluorescence enhanced by metal organic frameworks. ACS Appl Mater. Interfaces. 2018;10:23647–56.
Yuan X, Yeow TJ, Zhang Q, Lee JY, Xie J. Highly luminescent Ag+ nanoclusters for Hg2+ ion detection. Nanoscale. 2012;4:1968–71.
Yuan Z, Cai N, Du Y, He Y, Yeung ES. Sensitive and selective detection of copper ions with highly stable polyethyleneimine-protected silver nanoclusters. Anal Chem. 2014;86:419–26.
Li D, Qiao Z, Yu Y, Tang J, He X, Shi H, et al. In situ fluorescence activation of DNA–silver nanoclusters as a label-free and general strategy for cell nucleus imaging. Chem Commun. 2018;54:1089–92.
Adhikari B, Banerjee A. Facile synthesis of water-soluble fluorescent silver nanoclusters and HgII sensing. Chem Mater. 2010;22:4364–71.
Li D, Chen Z, Yang T. Fluorescence enhancement of DHLA protected gold nanoclusters in the presence of salt. New J Chem. 2016;40:3781–5.
Tang C, Feng H, Huang Y, Qian Z. Reversible luminescent nanoswitches based on aggregation-induced emission enhancement of silver nanoclusters for luminescence turn-on assay of inorganic pyrophosphatase activity. Anal Chem. 2017;89:4994–5002.
Ren SH, Liu SG, Ling Y, Li NB, Luo HQ. Fluorescence detection of melamine based on inhibiting Cu2+-induced disaggregation of red-emitting silver nanoclusters. Spectrochim Acta A. 2018;201:112–8.
Naaz S, Chowdhury P. Sunlight and ultrasound-assisted synthesis of photoluminescent silver nanoclusters: a unique ‘knock out’ sensor for thiophilic metal ions. Sens Actuators B. 2017;241:840–8.
Bayen SP, Mondal MK, Naaz S, Mondal SK, Chowdhury P. Design and sonochemical synthesis of water-soluble fluorescent silver nanoclusters for Hg2+ sensing. J Environ Chem Eng. 2016;4:1110–6.
Lakowitz JR. Principles of fluorescence spectroscopy. 3rd ed. New York: Springer; 2006.
Feng L, Sun Z, Liu H, Liu M, Jiang Y, Fan C, et al. Silver nanoclusters with enhanced fluorescence and specific ion recognition capability triggered by alcohol solvents: a highly selective fluorimetric strategy for detecting iodide ions in urine. Chem Commun. 2017;53:9466–9.
Sung TW, Lo YL, Chang IL. Highly sensitive and selective fluorescence probe for Cr3+ ion detection using water-soluble CdSe QDs. Sens Actuators B. 2014;202:1349–56.
Cheng Z. Studies on the interaction between scopoletin and two serum albumins by spectroscopic methods. J Lumin. 2012;132:2719–29.
Mei J, Leung NL, Kwok RT, Lam JW, Tang BZ. Aggregation-induced emission: together we shine, united we soar! Chem Rev. 2015;115:11718–940.
Naaz S, Poddar S, Bayen SP, Mondal MK, Roy D, Mondal SK, et al. Tenfold enhancement of fluorescence quantum yield of water soluble silver nanoclusters for nano-molar level glucose sensing and precise determination of blood glucose level. Sens Actuators B. 2018;255:332–40.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (no. 21675131) and the Natural Science Foundation of Chongqing (no. CSTC-2015jcyjB50001).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 599 kb)
Rights and permissions
About this article
Cite this article
Ren, S.H., Liu, S.G., Ling, Y. et al. Fabrication of silver nanoclusters with enhanced fluorescence triggered by ethanol solvent: a selective fluorescent probe for Cr3+ detection. Anal Bioanal Chem 411, 3301–3308 (2019). https://doi.org/10.1007/s00216-019-01796-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-019-01796-0