Abstract
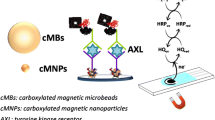
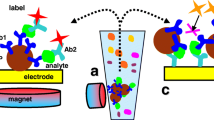
The evaluation of interaction between small molecules and protein is an important step in the discovery of new drugs and to study complex biological systems. In this work, an alternative method was presented to evaluate small-molecule–protein interaction by using ligand capture by protein-coated magnetic particles (MPs) and disposable electrochemical cells. The interaction study was conducted using [10]-gingerol from ginger rhizome and a transmembrane protein αVβ3 integrin. Initially, the electrochemical behavior of the natural compound [10]-gingerol was evaluated with the disposable carbon-based electrodes and presented an irreversible oxidation process controlled by diffusion. The analytical curve for [10]-gingerol was obtained in the range of 1.0 to 20.0 μmol L−1, with limit of detection of 0.26 μmol L−1. Then MPs coated with αVβ3 integrin were incubated with standard solutions and extracts of ginger rhizome for [10]-gingerol capture and separation. The bioconjugate obtained was dropped to the disposable electrochemical cells, keeping a permanent magnet behind the working electrode, and the binding process was evaluated by the electrochemical detection of [10]-gingerol. The assay method proposed was also employed to calculate the [10]-gingerol–αVβ3 integrin association constant, which was calculated as 4.3 × 107 M−1. The method proposed proved to be a good label-free alternative to ligand–protein interaction studies.

ᅟ





Similar content being viewed by others
References
McFedries A, Schwaid A, Saghatelian A. Methods for the elucidation of protein-small molecule interactions. Chem Biol. 2013;20:667–73. https://doi.org/10.1016/j.chembiol.2013.04.008.
Li X, Wang X, Snyder M. Systematic investigation of protein-small molecule interactions. IUBMB Life. 2013;65:2–8. https://doi.org/10.1002/iub.1111.
Pei H, Zuo X, Zhu D, Huang Q, Fan C. Functional DNA nanostructures for theranostic applications. Acc Chem Res. 2014;47:550–9. https://doi.org/10.1021/ar400195t.
Schenkman JB, Jansson I, Lvov Y, Rusling JF, Boussaad S, Tao NJ. Charge-dependent sidedness of cytochrome P450 forms studied by quartz crystal microbalance and atomic force microscopy. Arch Biochem Biophys. 2001;385:78–87. https://doi.org/10.1006/abbi.2000.2140.
Pei H, Lu N, Wen Y, Song S, Liu Y, Yan H, et al. A DNA nanostructure-based biomolecular probe carrier platform for electrochemical biosensing. Adv Mater. 2010;22:4754–8. https://doi.org/10.1002/adma.201002767.
Pei H, Wan Y, Li J, Hu H, Su Y, Huang Q, et al. Regenerable electrochemical immunological sensing at DNA nanostructure-decorated gold surfaces. Chem Commun. 2011;47:6254. https://doi.org/10.1039/c1cc11660f.
Humphries JD, Byron A, Humphries MJ. Integrin ligands at a glance. J Cell Sci. 2006;119:3901 LP-3903.
Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10:9–22. https://doi.org/10.1038/nrc2748.
Beer AJ, Kessler H, Wester H-J, Schwaiger M. PET imaging of integrin αVβ3 expression. Theranostics. 2011;1:48–57.
Davis PJ, Glinsky GV, Lin HY, Leith JT, Hercbergs A, Tang HY, et al. Cancer cell gene expression modulated from plasma membrane integrin αvβ3 by thyroid hormone and nanoparticulate tetrac. Front Endocrinol (Lausanne). 2014;5:240. https://doi.org/10.3389/fendo.2014.00240.
Lin H-Y, Lansing L, Merillon J-M, Davis FB, Tang H-Y, Shih A, et al. Integrin alphaVbeta3 contains a receptor site for resveratrol. FASEB J. 2006;20:1742–4. https://doi.org/10.1096/fj.06-5743fje.
Belleri M, Ribatti D, Savio M, Stivala LA, Forti L, Tanghetti E, et al. alphavbeta3 integrin-dependent antiangiogenic activity of resveratrol stereoisomers. Mol Cancer Ther. 2008;7:3761–70. https://doi.org/10.1158/1535-7163.MCT-07-2351.
Kim HI, Huang H, Cheepala S, Huang S, Chung J. Curcumin inhibition of integrin ( 6 4)-dependent breast cancer cell motility and invasion. Cancer Prev Res. 2008;1:385–91. https://doi.org/10.1158/1940-6207.CAPR-08-0087.
Davis PJ, Mousa SA, Cody V, Tang H-Y, Lin H-Y. Small molecule hormone or hormone-like ligands of integrin αVβ3: implications for cancer cell behavior. Horm Cancer. 2013;4:335–42. https://doi.org/10.1007/s12672-013-0156-8.
Yang GX, Li X, Snyder M. Investigating metabolite-protein interactions: an overview of available techniques. Methods. 2012;57:459–66. https://doi.org/10.1016/j.ymeth.2012.06.013.
Terstappen GC, Schlüpen C, Raggiaschi R, Gaviraghi G. Target deconvolution strategies in drug discovery. Nat Rev Drug Discov. 2007;6:891–903. https://doi.org/10.1038/nrd2410.
Myszka DG. Analysis of small-molecule interactions using Biacore S51 technology. Anal Biochem. 2004;329:316–23. https://doi.org/10.1016/j.ab.2004.03.028.
Cooper DR, Porebski PJ, Chruszcz M, Minor W. X-ray crystallography: assessment and validation of protein-small molecule complexes for drug discovery. Expert Opin Drug Discov. 2011;6:771–82. https://doi.org/10.1517/17460441.2011.585154.
Krell T, Lacal J, García-Fontana C, Silva-Jiménez H, Rico-Jiménez M, Lugo AC, Darias JAR, Ramos J-L. Characterization of molecular interactions using isothermal titration calorimetry. In: Methods in molecular biology (Clifton, NJ). 2014; pp 193–203.
Afsahi S, Lerner MB, Goldstein JM, Lee J, Tang X, Bagarozzi DA, et al. Novel graphene-based biosensor for early detection of Zika virus infection. Biosens Bioelectron. 2018;100:85–8. https://doi.org/10.1016/J.BIOS.2017.08.051.
Gault J, Donlan JAC, Liko I, Hopper JTS, Gupta K, Housden NG, et al. High-resolution mass spectrometry of small molecules bound to membrane proteins. Nat Methods. 2016;13:333–6. https://doi.org/10.1038/nmeth.3771.
Hernández-Hernández AA, Álvarez-Romero GA, Contreras-López E, Aguilar-Arteaga K, Castañeda-Ovando A. Food analysis by microextraction methods based on the use of magnetic nanoparticles as supports: recent advances. Food Anal Methods. 2017;10:2974–93.
De Oliveira RAG, Materon EM, Melendez ME, Carvalho AL, Faria RC. Disposable microfluidic Immunoarray device for sensitive breast cancer biomarker detection. ACS Appl Mater Interfaces. 2017;9:27433–40. https://doi.org/10.1021/acsami.7b03350.
Modh H, Scheper T, Walter J-G. Aptamer-modified magnetic beads in biosensing. Sensors. 2018;18:1041. https://doi.org/10.3390/s18041041.
Uliana CV, Peverari CR, Afonso AS, Cominetti MR, Faria RC. Fully disposable microfluidic electrochemical device for detection of estrogen receptor alpha breast cancer biomarker. Biosens Bioelectron. 2018;99:156–62. https://doi.org/10.1016/j.bios.2017.07.043.
Marszałł MP, Buciński A. A protein-coated magnetic beads as a tool for the rapid drug-protein binding study. J Pharm Biomed Anal. 2010;52:420–4. https://doi.org/10.1016/j.jpba.2009.06.023.
Yasuda M, Wilson DR, Fugmann SD, Moaddel R. Synthesis and characterization of SIRT6 protein coated magnetic beads: identification of a novel inhibitor of SIRT6 deacetylase from medicinal plant extracts. Anal Chem. 2011;83:7400–7. https://doi.org/10.1021/ac201403y.
Ji L, Wu J-H, Luo Q, Li X, Zheng W, Zhai G, et al. Quantitative mass spectrometry combined with separation and enrichment of phosphopeptides by titania coated magnetic mesoporous silica microspheres for screening of protein kinase inhibitors. Anal Chem. 2012;84:2284–91. https://doi.org/10.1021/ac202897u.
Xu Y, Wang E. Electrochemical biosensors based on magnetic micro/nano particles. Electrochim Acta. 2012;84:62–73. https://doi.org/10.1016/j.electacta.2012.03.147.
Zeidan N, Su H, Aitken M, Gunning PT, Kerman K. Magnetic bead-based electrochemical detection of interaction between epigallocatechin-3-gallate and STAT proteins. Anal Methods. 2015;7:3566–9. https://doi.org/10.1039/C5AY00169B.
Joo J-H, Hong S-S, Cho Y-R, Seo D-W. 10-Gingerol inhibits proliferation and invasion of MDA-MB-231 breast cancer cells through suppression of Akt and p38MAPK activity. Oncol Rep. 2016;35:779–84.
da Silva JA, Becceneri AB, Mutti HS, Martin ACBM, Silva MF das GF, Fernandes JB, et al. Purification and differential biological effects of ginger-derived substances on normal and tumor cell lines. J Chromatogr B. 2012;903:157–62. https://doi.org/10.1016/j.jchromb.2012.07.013.
Fuzer AM, Lee S-Y, Mott JD, Cominetti MR. [10]-Gingerol reverts malignant phenotype of breast cancer cells in 3D culture. J Cell Biochem. 2017;118:2693–9. https://doi.org/10.1002/jcb.25906.
Martin ACBM, Fuzer AM, Becceneri AB, da Silva JA, Tomasin R, Denoyer D, et al. [10]-gingerol induces apoptosis and inhibits metastatic dissemination of triple negative breast cancer in vivo. Oncotarget. 2017;8:72260–71. https://doi.org/10.18632/oncotarget.20139.
Afonso AS, Uliana CV, Martucci DH, Faria RC. Simple and rapid fabrication of disposable carbon-based electrochemical cells using an electronic craft cutter for sensor and biosensor applications. Talanta. 2016;146:381–7. https://doi.org/10.1016/j.talanta.2015.09.002.
Chaisiwamongkhol K, Ngamchuea K, Batchelor-McAuley C, Compton RG. Electrochemical detection and quantification of gingerol species in ginger (Zingiber officinale) using multiwalled carbon nanotube modified electrodes. Analyst. 2016;141:6321–8. https://doi.org/10.1039/C6AN02254E.
Crespo JG, João G, Böddeker KW Karl W. Membrane processes in separation and purification.
Bard AJ, Faulkner LR. Electrochemical methods : fundamentals and applications. Hoboken: Wiley; 2001.
Garbellini GS, Rocha-Filho RC, Fatibello-Filho O. Voltammetric determination of ciprofloxacin in urine samples and its interaction with dsDNA on a cathodically pretreated boron-doped diamond electrode. Anal Methods. 2015;7:3411–8. https://doi.org/10.1039/C5AY00625B.
Arkin MR, Wells JA. Small-molecule inhibitors of protein–protein interactions: progressing towards the dream. Nat Rev Drug Discov. 2004;3:301–17. https://doi.org/10.1038/nrd1343.
Kuriyan J, Konforti B, Wemmer D. The molecules of life : physical and chemical principles. New York: Taylor & Francis Group; 2013.
Vachali PP, Li B, Bartschi A. Surface plasmon resonance (SPR)-based biosensor technology for the quantitative characterization of protein–carotenoid interactions. Arch Biochem Biophys. 2015;572:66–72. https://doi.org/10.1016/J.ABB.2014.12.005.
Cheng XR, Wallace GQ, Lagugné-Labarthet F, Kerman K. Au nanostructured surfaces for electrochemical and localized surface plasmon resonance-based monitoring of α-Synuclein–small molecule interactions. ACS Appl Mater Interfaces. 2015;7:4081–8. https://doi.org/10.1021/am507972b.
Zhuo R, Liu H, Liu N, Wang Y. Ligand fishing: a remarkable strategy for discovering bioactive compounds from complex mixture of natural products. Molecules. 2016;21:1516.
Funding
This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, No. 150569/2016-5), Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, No. 2015/19890-1, No. 2014/22401-0, and No. 2015/24940-8), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, No. 01).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 462 kb)
Rights and permissions
About this article
Cite this article
Uliana, C.V., de Oliveira, T.R., Cominetti, M.R. et al. Label-free evaluation of small-molecule–protein interaction using magnetic capture and electrochemical detection. Anal Bioanal Chem 411, 2111–2119 (2019). https://doi.org/10.1007/s00216-019-01636-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-019-01636-1