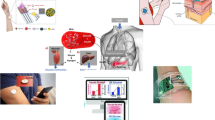
Abstract
A blood glucose meter is an electronic medical device used for determining the concentration of glucose in blood. These meters have undergone five phases of development: washed blood glucose meters, wiped blood glucose meters, colorimetric blood glucose meters, electrochemical blood glucose meters, and micro, multiple site blood glucose meters. Thanks to their speed, portability, low cost, and easy operation, blood glucose meters have been widely available for use in clinical diagnosis. Recently, coupling of target recognition elements (antibody–antigen recognition, nucleic acid hybridization, enzyme recognition, and click chemistry) with signal transduction and amplification strategies (glucose-generating enzymes, nicotinamide adenine dinucleotide (NADH)-generating enzymes, encapsulated glucose, nanomaterials, and cyclic amplification of DNA) has allowed various targets to be determined via the relationship between the signal of the blood glucose meter and the concentration of targets. In this paper, a brief review of the development and mechanism of blood glucose meters is given first. Then, more details on the application of blood glucose meters in analysis are described, including biomedical analysis, food analysis, and environmental analysis. Finally, the prospect of future development of blood glucose meters is also discussed.

ᅟ








Similar content being viewed by others
References
Clerc O, Greub G. Routine use of point-of-care tests: usefulness and application in clinical microbiology. Clin Microbiol Infec. 2010;16(8):1054–61.
Gubala V, Harris LF, Ricco AJ, Tan MX, Williams DE. Point of care diagnostics: status and future. Anal Chem. 2012;84(2):487–515.
Bissell M, Sanfilippo F. Empowering patients with point-of-care testing. Trends Biotechnol. 2002;20(6):269–70.
Liu F. Quantitative DNA and myoglobin detection using portable POCT analyzer. Masteral dissertation of Hunan University; 2013.
Lee SR, Lee YT, Sawada K, Takao H, Ishida M. Development of a disposable glucose biosensor using electroless-plated Au/Ni/copper low electrical resistance electrodes. Biosens Bioelectron. 2008;24(3):410–4.
Leland C, Clark J, Champ L. Electrode systems for continuous monitoring in cardiovascular surgery. Ann N Y Acad Sci. 1962;102:29–45.
Montagnana M, Caputo M, Giavarina D, Lippi G. Overview on self-monitoring of blood glucose. Clin Chim Acta. 2009;402:7–13.
Clarke SF, Foster JR. A history of blood glucose meters and their role in self-monitoring of diabetes mellitus. Br J Biomed Sci. 2012;69(2):83–93.
Free AH, Adams EC, Kercher ML, Free HM, Cook MH. Simple specific test for urine glucose. Clin Chem. 1957;3(3):163–8.
Mendosa D. History of blood glucose meters: transcripts of interviews, 2006 (www.mendosa.com/history/htm).
Leroux ML, Desjardine PRE. Ward level evaluation of the “one touch” glucose meter. Clin Chem. 1988;34(9):1928–9.
Burritt MF. Current analytical approaches to measuring blood analytes. Clin Chem. 1990;36:1562–6.
Batki AD, Thomason HL, Holder R, Nayyar P, GHG Thorpe. Medical Devices Agency-MDA02002. Medical Devices Agency evaluation report. 2010.
Wang J. Electrochemical glucose biosensors. Chem Rev. 2008;108(2):814–25.
Newman JD, Turner AP. Home blood glucose biosensors: a commercial perspective. Biosens Bioelectron. 2005;20(12):2435–53.
Hu J, Xie QJ, Yang DW, Xiao HL, Fu YC, Tan YM, et al. Recent advances in electrochemical glucose biosensors: a review. RSC Adv. 2013;3(14):4473–91.
Yoo EH, Lee SY. Glucose biosensors: an overview of use in clinical practice. Sensors. 2010;10:4558–76.
Boguslavsky LI, Geng L, Kovalev IP, Sahni SK, Skotheim TA, Laurinavicius B, et al. Amperometric thin film biosensors based on glucose dehydrogenase and toluidine blue O as catalyst for NADH electrooxidation. Biosens Bioelectron. 1995;10(1):693–704.
Tsujimura S, Kojima S, Kano K, Ikeda T, Sato M, Sanada H, et al. Novel FAD-dependent glucose dehydrogenase for a dioxygen-insensitive glucose biosensor. Biosci Biotechnol Biochem. 2006;70:654–9.
International Organization for Standardization. Geneva: ISO, 15197–2011.
Huang ZP, Tian XT, Wu WH, Zhang M. Application of peptide-based fluorescent probes in protein detection. Sci Chin Chem. 2013;43(8):1013–21.
Xiang Y, Lu Y. Using personal glucose meters and functional DNA sensors to quantify a variety of analytical targets. Nat Chem. 2011;3:697–703.
Langer Q. Portable glucose detector developed to quantify a range of analytes. Bioanalysis. 2011;3(16):1806–7.
Xiang Y, Lu Y. Portable and quantitative detection of protein biomarkers and small molecular toxins using antibodies and ubiquitous personal glucose meters. Anal Chem. 2012;84:4174–8.
Su J, Xu J, Chen Y, Xiang Y, Yuan R, Chai YQ. Personal glucose sensor for point-of-care early cancer diagnosis. Chem Commun. 2012;48:6909–11.
Ma XM, Chen ZH, Zhou J, Weng W, Zheng O, Lin ZY, et al. Aptamer-based portable biosensor for platelet-derived growth factor-BB (PDGF-BB) with personal glucose meter readout. Biosens Bioelectron. 2014;55:412–6.
Fu XH, Feng XR, Xu K, Huang R. A portable and quantitative enzyme immunoassay of neuron-specific enolase with a glucometer readout. Anal Methods. 2014;6:2233–8.
Chen JH, Wu W, Zeng LW. A universal glucometer-based biosensor for portable and quantitative detection of transcription factors. Anal Methods. 2014;6:4840–4.
Fu XH, Xu K, Ye J, Chen J, Feng XY. Glucoamylase-labeled nanogold flowers for in situ enhanced sensitivity of a glucometer-based enzyme immunoassay. Anal Methods. 2015;7:507–12.
Lin JS, Tang DP. Glucometer-based signal readout for a portable low-cost electrochemical immunoassay using branched platinum nanowires. Anal Methods. 2016;8:4069–74.
Lin Q, Liu D, Yan JM, Qiao Z, Zhong YX, Yan JW, et al. Enzyme-encapsulated liposome-linked immunosorbent assay enabling sensitive personal glucose meter readout for portable detection of disease biomarkers. ACS Appl Mater Interfaces. 2016;8:6890–7.
Wu S, Chen JS, Tian YJ, Tang XM, Li W, Li JM. Biofunctionalized dendritic polyaniline nanofiber for in situ amplified glucometer-based enzyme immunoassay of tumor marker. Anal Methods. 2015;7(5):1843–8.
Hun X, Xu YQ, Luo XL. Peptide-based biosensor for the prostate-specific antigen using magnetic particle-bound invertase and a personal glucose meter for readout. Microchim Acta. 2015;182:1669–75.
Zhu X, Zheng HY, Xu HF, Lin RL, Han YJ, Yang GD, et al. A reusable and portable immunosensor using personal glucose meter as transducer. Anal Methods. 2014;6:5264–8.
Zhao YT, Du D, Lin YH. Glucose encapsulating liposome for signal amplification for quantitative detection of biomarkers with glucometer readout. Biosens Bioelectron. 2015;72:348–54.
Wang Q, Liu F, Yang XH, Wang KM, Wang H, Deng X. Sensitive point-of-care monitoring of cardiac biomarker myoglobin using aptamer and ubiquitous personal glucose meter. Biosens Bioelectron. 2015;64:161–4.
Xiang Y, Lan T, Lu Y. Using the widely available blood glucose meter to monitor insulin and HbA1c. J Diabetes Sci Technol. 2014;8(4):855–8.
Zhu X, Kou F, Xu H, Lin L, Yang G, Lin Z. A highly sensitive aptamer-immunoassay for vascular endothelial growth factor coupled with portable glucose meter and hybridization chain reaction. Sensor Actuat B-Chem. 2017;253:660–5.
Taebi S, Keyhanfar M, Noorbakhsh A. A novel method for sensitive, low-cost and portable detection of hepatitis B surface antigen using a personal glucose meter. J Immunol Methods. 2018;458:26–32.
Ismail NF, Lim TS. Site-specific scFv labelling with invertase via Sortase a mechanism as a platform for antibody-antigen detection using the personal glucose meter. Sci Rep. 2016;6:19338–51.
Hong L, Zhou F, Shi D, Zhang X, Wang GF. Portable aptamer biosensor of platelet-derived growth factor-BB using a personal glucose meter with triply amplified. Biosens Bioelectron. 2017;95:152–9.
Huang Y, Duan XF, Cui Y, Lauhon LJ, Kim KH, Lieber CM. Logic gates and computation from assembled nanowire building blocks. Science. 2001;294:1313–7.
Hou L, Zhu CL, Wu XP, Chen GN, Tang DP. Bioresponsive controlled release from mesoporous silica nanocontainers with glucometer readout. Chem Commun. 2014;50:1441–3.
Yan L, Zhu Z, Zou Y, Huang YS, Liu DW, Jia SS, et al. Target-responsive “sweet” hydrogel with glucometer readout for portable and quantitative detection of non-glucose targets. J Am Chem Soc. 2013;135(10):3748–51.
Zhu X, Sarwar M, Yue Q, Chen C, Li C. Biosensing of DNA oxidative damage: a model of using glucose meter for non-glucose biomarker detection. Int J Nanomedicine. 2017;12:979–87.
Xiang Y, Lu Y. Using commercially available personal glucose meters for portable quantification of DNA. Anal Chem. 2012;84:1975–80.
Xu J, Jiang BY, Xie JQ, Xiang Y, Yuan R, Chai YQ. Sensitive point-of-care monitoring of HIV related DNA sequences with a personal glucometer. Chem Commun. 2012;48:10733–5.
Xu XT, Liang KY, Zeng JY. Portable and sensitive quantitative detection of DNA using personal glucose meters and exonuclease III-assisted signal amplification. Analyst. 2014;139:4982–6.
Xu XT, Liang KY, Zeng JY. Portable and sensitive quantitative detection of DNA based on personal glucose meters and isothermal circular strand-displacement polymerization reaction. Biosens Bioelectron. 2015;64:671–5.
Wang Q, Wang H, Yang XH, Wang KM, Liu F, Zhao Q, et al. Multiplex detection of nucleic acids using a low cost microfluidic chip and a personal glucose meter at the point-of-care. Chem Commun. 2014;50:3824–6.
Gu Y, Zhang T, Huang Z, Hu S, Zhao W, Xu J, et al. An exploration of nucleic acid liquid biopsy using a glucose meter. Chem Sci. 2018;9:3517–22.
Wang YM, Lu MH, Zhu JH, Tian SF. Wrapping DNA-gated mesoporous silica nanoparticles for quantitative monitoring of telomerase activity with glucometer readout. J Mater Chem B. 2014;2:5847–52.
Zhu X, Xu HF, Lin RL, Yang GD, Lin ZY, Chen GN. Sensitive and portable detection of telomerase activity in HeLa cells using the personal glucose meter. Chem Commun. 2014;50:7897–9.
Wang WJ, Huang S, Li JJ, Rui K, Zhang JR, Zhu JJ. Coupling a DNA-based machine with glucometer readouts for amplified detection of telomerase activity in cancer cells. Sci Rep. 2016;6:23504.
Sun AL, Jia FC, Zhang YF, Wang XN. Gold nanocluster-encapsulated glucoamylase as a biolabel for sensitive detection of thrombin with glucometer readout. Microchim Acta. 2015;182:1169–75.
Yang WX, Lu XH, Wang YC, Sun SJ, Liu CH, Li ZP. Portable and sensitive detection of protein kinase activity by using commercial personal glucose meter. Sensor Actuat B-Chem. 2015;210:508–12.
Deng H, Peng S, Gao Z. Highly sensitive detection of M.SssI DNA methyltransferase activity using a personal glucose meter. Anal Bioanal Chem. 2016;408:5867–72.
Zhang Y, Xue Q, Liu J, Wang H. Magnetic bead-liposome hybrids enable sensitive and portable detection of DNA methyltransferase activity using personal glucose meter. Biosens Bioelectron. 2017;87:537–44.
Mohapatra H, Phillips ST. Reagents and assay strategies for quantifying active enzyme analytes using a personal glucose meter. Chem Commun. 2013;49:6134–6.
Zhang JJ, Xiang Y, Novak DE, Hoganson GE, Zhu JJ, Lu Y. Using a personal glucose meter and alkaline phosphatase for point-of-care quantification of galactose-1-phosphate uridyltransferase in clinical galactosemia diagnosis. Chem Asian J. 2015;10:2221–7.
Zhang JJ, Xiang Y, Wang M, Basu A, Lu Y. Dose-dependent response of personal glucose meters to nicotinamide coenzymes: applications to point-of-care diagnostics of many non-glucose targets in a single step. Angew Chem Int Ed. 2016;55:732–6.
Wang Q, Wang H, Yang XH, Wang KM, Liu RJ, Li Q, et al. A sensitive one-step method for quantitative detection of α-amylase in serum and urine using a personal glucose meter. Analyst. 2015;140:1161–5.
Bai J, Liu L, Han Y, Jia C, Liang C. One-step detection of hexokinase activity using personal glucose meter. Anal Methods. 2018;10(18):2075–80.
Chen Y, Yi H, Xiang Y, Ruan R. Commercial glucometer as signal transducer for simple evaluation of DNA methyltransferase activity and inhibitors screening. Anal Chim Acta. 2018;1001:18–23.
Du Y, Hughes RA, Bhadra S, Jiang YS, Ellington AD, Li BL. A sweet spot for molecular diagnostics: coupling isothermal amplification and strand exchange circuits to glucometers. Sci Rep. 2014;5:11039–54.
Hun X, Xu YQ, Xie GL, Luo XL. Aptamer biosensor for highly sensitive and selective detection of dopamine using ubiquitous personal glucose meters. Sensor Actuat B-Chem. 2015;209:596–601.
Zhang JJ, Shen Z, Xiang Y, Lu Y. Integration of solution-based assays onto lateral flow device for one-step quantitative point-of-care diagnostics using personal glucose meter. ACS Sens. 2016;9:1091–6.
Tan Q, Zhang R, Kong R, Kong W, Zhao W, Qu F. Detection of glutathione based on MnO2 nanosheet-gated mesoporous silica nanoparticles and target induced release of glucose measured with a portable glucose meter. Microchim Acta. 2018;185:44.
Jia F. The study on aptasensor of food-borne pathogens based on grapheme oxide nanomaterials. Masteral Dissertation of Jiangnan University, 2014.
Chen Y. Research of rapid detection of food safety microorganism using personal blood glucose meter. Masteral Dissertation of Zhejiang Gongshang University, 2014.
Joo J, Kwon D, Shin HH, Park KH, Cha HJ, Jeon S. A facile and sensitive method for detecting pathogenic bacteria using personal glucose meters. Sensor Actuat B-Chem. 2013;188:1250–4.
Chavali R, Gunda NSK, Naicker S, Mitra SK. Detection of Escherichia coli in potable water using personal glucose meters. Anal Methods. 2014;6:6223–7.
Wang ZZ, Chen Z, Gao N, Ren J, Qu X. Transmutation of personal glucose meters into portable and highly sensitive microbial pathogen detection platform. Small. 2015;11(37):4970–5.
Wan Y, Qi P, Zeng Y, Sun Y, Zhang D. Invertase-mediated system for simple and rapid detection of pathogen. Sensor Actuat B-Chem. 2016;233:454–8.
Huang H, Zhao G, Dou W. Portable and quantitative point-of-care monitoring of Escherichia coli O157:H7 using a personal glucose meter based on immunochromatographic assay. Biosens Bioelectron. 2018;107:266–71.
Bacaloni A, Cavaliere C, Cucci F, Foglia P, Samperi R, Laganà A. Determination of aflatoxins in hazelnuts by various sample preparation methods and liquid chromatography–tandem mass spectrometry. J Chromatogr A. 2008;1179(2):182–9.
Tang D, Lin Y, Zhou Q, Lin Y, Li P, Niessner R, et al. Low-cost and highly sensitive immunosensing platform for aflatoxins using one-step competitive displacement reaction mode and portable glucometer-based detection. Anal Chem. 2014;86:11451–8.
Tang J, Huan Y, Liu H, Zhang C, Tang D. Novel glucometer-based immunosensing strategy suitable for complex systems with signal amplification using surfactant-responsive cargo release from glucose-encapsulated liposome nanocarriers. Biosens Bioelectron. 2016;79:508–14.
Yang X, Shi D, Zhu S, Wang B, Zhang X, Wang G. Portable aptasensor of aflatoxin B1 in bread based on a personal glucose meter and DNA walking machine. ACS Sens. 2018; https://doi.org/10.1021/acssensors.8b00304.
Gu C, Long F, Zhou X, Shi H. Portable detection of ochratoxin a in red wine based on a structure-switching aptamer using a personal glucometer. RSC Adv. 2016;6:29563–8.
Zhang Y, Zheng N, Wen F, Zhang YD, Li FD, Wang JQ. Method research of quantitative detection for ochratoxin A by aptasensor combining portable glucose meter. J Agr Sci Technol. 2016;18(1):182–6.
Gao Z, Tang D, Xu M, Chen G, Yang H. Nanoparticle-based pseudo hapten for target-responsive cargo release from a magnetic mesoporous silica nanocontainer. Chem Commun. 2014;50:6256–8.
Zhang CY, Zhang MW, Li HK, Li Y, Song PH, Guo JJ, et al. Gold nanoparticles-based colorimetric sensing of melamine in milk and eggs. Chin J Anal Chem. 2012;3:386–90.
Gu C, Lan T, Shi H, Lu Y. Portable detection of melamine in milk using a personal glucose meter based on an in vitro selected structure-switching aptamer. Anal Chem. 2015;87(15):7676–82.
Li F, Zhang R, Kang H, Hu Y, Liu Y, Zhu J. Facile and sensitive detection of clenbuterol in pork using a personal glucose meter. Anal Methods. 2017;9:6507–12.
Chen S, Zhang JB, Gan N, Hu FT, Li T, Cao Y, et al. An on-site immunosensor for ractopamine based on a personal glucose meter and using magnetic β-cyclodextrin-coated nanoparticles for enrichment, and an invertase-labeled nanogold probe for signal amplification. Microchim Acta. 2015;182:815–22.
Chen S, Gan N, Zhang H, Hu F, Li T, Cui H, et al. A portable and antibody-free sandwich assay for determination of chloramphenicol in food based on a personal glucose meter. Anal Bioanal Chem. 2015;407:2499–507.
Kwon D, Lee H, Yoo H, Hwang J, Lee D, Jeon S. Facile method for enrofloxacin detection in milk using a personal glucose meter. Sensor Actuat B-Chem. 2018;254:935–9.
Zhou J, Xu K, Zhou P, Zheng O, Lin Z, Guo L, et al. A portable chemical sensor for histidine based on the strategy of click chemistry. Biosens Bioelectron. 2014;51:386–90.
Zhang XH, Zhou TR, Chen X. Applications of metal nanoclusters in environmental monitoring. Chin J Anal Chem. 2015;43(9):1296–305.
Liu HY. Synthesis and analytical application of the gold nanomaterials. Doctoral Dissertation of Nanjing University, 2011.
Zhang J, Tang Y, Lv J, Fang S, Tang D. Glucometer-based quantitative determination ofHg(II) using gold particle encapsulated invertase and strong thymine-Hg(II)-thymine interaction for signal amplification. Microchim Acta. 2015;182:1153–9.
Qiu Z, Shu J, Jin G, Xu M, Wei Q, Chen G, et al. Invertase-labeling gold-dendrimer for in situ amplified detection mercury(II) with glucometer readout and thymine–Hg2+–thymine coordination chemistry. Biosens Bioelectron. 2016;77:681–6.
Huang S, Wang W, Cheng F, Yao H, Zhu J. Highly sensitive detection of mercury ion based on T-rich DNA machine using portable glucose meter. Sensor Actuat B-Chem. 2017;242:347–54.
Xu X, Liang K, Zeng J. Highly sensitive and portable mercury(II) ion sensor using personal glucose meter. Anal Methods. 2015;7:81–5.
Liang X, Wang L, Wang D, Zeng L, Fang Z. Portable and quantitative monitoring of mercury ions using DNA-gated mesoporous silica nanoparticles using a glucometer readout. Chem Commun. 2016;52:2192–4.
Li W, Yang Y, Chen J, Zhang Q, Wang Y, Wang F, et al. Detection of lead(II) ions with a DNAzyme and isothermal strand displacement signal amplification. Biosens Bioelectron. 2014;53:245–9.
Xiang Y, Lu Y. An invasive DNA approach toward a general method for portable quantification of metal ions using a personal glucose meter. Chem Commun. 2013;49:585–7.
Fu L, Zhuang J, Lai W, Que X, Lu M, Tang D. Portable and quantitative monitoring of heavy metal ions using DNAzyme-capped mesoporous silica nanoparticles with a glucometer readout. J Mater Chem B. 2013;1:6123–8.
Zhang J, Tang Y, Teng L, Lu M, Tang D. Low-cost and highly efficient DNA biosensor for heavy metal ion using specific DNAzyme-modified microplate and portable glucometer-based detection mode. Biosens Bioelectron. 2015;68:232–8.
Liao J, Li H. Target-induced DNAzyme cleavage accompanying bioactive enzymatic assembly with glucometer readout for quantitative monitoring of lead ion. Chem Lett. 2014;43(10):1599–600.
Wang ZH, Gao YL, Zhang FF, Xia YZ, Li YH. Applications of quantum dot in analysis and detection. J Anal Sci. 2012;28(1):119–25.
Su J, Xu J, Chen Y, Xiang Y, Yuan R, Chai Y. Sensitive detection of copper(II) by a commercial glucometer using click chemistry. Biosens Bioelectron. 2013;45:219–22.
Ming J, Fan W, Jiang T, Wang Y, Lv Z. Portable and sensitive detection of copper(II) ion based on personal glucose meters and a ligation DNAzyme releasing strategy. Sensor Actuat B-Chem. 2017;240:1091–8.
Fang D, Gao G, Yu Y, Shen J, Zhi J. Adaptive use of a personal glucose meter (PGM) for acute biotoxicity assessment based on the glucose consumption of microbes. Analyst. 2016;141:3004–11.
Acknowledgements
This study was financed by the NSFC (21405029 and 51173035), Science and Technology Program of Zhejiang Province (LGF18H200005), Young Talent Development Project of Zhejiang Science and Technology Association (2016YCGC007), Medical and Health Technology Development Program of Zhejiang Province (2017KY533), the Social Development Project of Hangzhou (20160533B70).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Zhang, L., Gu, C., Ma, H. et al. Portable glucose meter: trends in techniques and its potential application in analysis. Anal Bioanal Chem 411, 21–36 (2019). https://doi.org/10.1007/s00216-018-1361-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-018-1361-7