Abstract
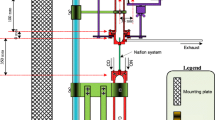
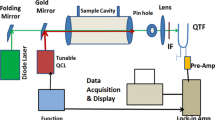
Triacetone triperoxide (TATP) is frequently used in improvised explosive devices because of its ease of manufacture and tremendous explosive force. In this paper, we describe a new method for detection of TATP, thermal decomposition peroxy radical chemical amplification cavity ring-down spectroscopy (TD-PERCA-CRDS). In this method, air is sampled through a heated inlet to which ~ 1 ppmv nitric oxide (NO) is added. To verify the purity of synthetic standards, the mid-infrared spectrum of TATP vapor was recorded. The thermal decomposition of TATP is shown to produce radicals which oxidize NO to nitrogen dioxide (NO2), whose concentration increase is monitored by optical absorption at 405 nm using a blue diode laser CRDS. The sensitivity could be improved through addition of ~ 1% ethane (C2H6), which fuels catalytic conversion of NO to NO2. The limit of detection of TD-PERCA-CRDS with respect to TATP is 22 pptv (1 s data), approximately six orders of magnitude below TATP’s saturation vapor pressure. Insights into the mechanism of TATP thermal decomposition, TD-PERCA-CRDS interferences, and the suitability of TD-PERCA-CRDS as a peroxy radical explosive detection method at security check points are discussed.




Similar content being viewed by others
References
Oxley JC, Smith JL, Chen H. Decomposition of a multi-peroxidic compound: triacetone triperoxide (TATP). Propellants Explos Pyrotech. 2002;27(4):209–16. https://doi.org/10.1002/1521-4087(200209)27:4<209::aid-prep209>3.0.co;2-j.
Dubnikova F, Kosloff R, Almog J, Zeiri Y, Boese R, Itzhaky H, et al. Decomposition of triacetone triperoxide is an entropic explosion. J Am Chem Soc. 2005;127(4):1146–59. https://doi.org/10.1021/ja0464903.
Ewing RG, Waltman MJ, Atkinson DA, Grate JW, Hotchkiss PJ. The vapor pressures of explosives. TrAC Trends Anal Chem. 2013;42(0):35–48. https://doi.org/10.1016/j.trac.2012.09.010.
Mbah J, Knott D, Steward S. Thermogravimetric study of vapor pressure of TATP synthesized without recrystallization. Talanta. 2014;129:586–93. https://doi.org/10.1016/j.talanta.2014.06.031.
Schulte-Ladbeck R, Vogel M, Karst U. Recent methods for the determination of peroxide-based explosives. Anal Bioanal Chem. 2006;386(3):559–65. https://doi.org/10.1007/s00216-006-0579-y.
Bulatov V, Reany O, Grinko R, Schechter I, Keinan E. Time-resolved, laser initiated detonation of TATP supports the previously predicted non-redox mechanism. Phys Chem Chem Phys. 2013;15(16):6041–8. https://doi.org/10.1039/c3cp44662j.
Hiyoshi RI, Nakamura J, Brill TB. Thermal decomposition of organic peroxides TATP and HMTD by T-jump/FTIR spectroscopy. Propellants, Explosives, Pyrotechnics. 2007;32(2):127–34. https://doi.org/10.1002/prep.200700002.
Burkholder JB, Sander SP, Abbatt JPD, Barker JR, Huie RE, Kolb CE, et al. Chemical kinetics and photochemical data for use in atmospheric studies, Evaluation Number 18. Pasadena: National Aeronautics and Space Administration, Jet Propulsion Laboratory, California Institute of Technology; 2015.
Lightfoot PD, Cox RA, Crowley JN, Destriau M, Hayman GD, Jenkin ME, et al. Organic peroxy-radicals—kinetics, spectroscopy and tropospheric chemistry. Atmos Environm A. 1992;26(10):1805–961. https://doi.org/10.1016/0960-1686(92)90423-I.
Clemitshaw KC, Carpenter LJ, Penkett SA, Jenkin ME. A calibrated peroxy radical chemical amplifier for ground-based tropospheric measurements. J Geophys Res. 1997;102(D21):25405–16. https://doi.org/10.1029/97JD01902.
Cantrell CA, Shetter RE, Lind JA, McDaniel AH, Calvert JG, Parrish DD, et al. An improved chemical amplifier technique for peroxy radical measurements. J Geophys Res. 1993;98(D2):2897–909. https://doi.org/10.1029/92JD02842.
Atkinson R, Baulch DL, Cox RA, Crowley JN, Hampson RF, Hynes RG, et al. Evaluated kinetic and photochemical data for atmospheric chemistry: volume I—gas phase reactions of Ox, HOx, NOx and SOx species. Atmos Chem Phys. 2004;4:1461–738. https://doi.org/10.5194/acp-4-1461-2004.
Baulch DL, Cobos CJ, Cox RA, Frank P, Hayman G, Just T, et al. Summary table of evaluated kinetic data for combustion modeling: supplement 1. Combust Flame. 1994;98(1):59–79. https://doi.org/10.1016/0010-2180(94)90198-8.
Wood EC, Deming BL, Kundu S. Ethane-based chemical amplification measurement technique for atmospheric peroxy radicals. Environ Sci Technol Lett. 2016;4(1):15–9. https://doi.org/10.1021/acs.estlett.6b00438.
Paul D, Osthoff HD. Absolute measurements of total peroxy nitrate mixing ratios by thermal dissociation blue diode laser cavity ring-down spectroscopy. Anal Chem. 2010;82(15):6695–703. https://doi.org/10.1021/ac101441z.
Taha YM, Odame-Ankrah CA, Osthoff HD. Real-time vapor detection of nitroaromatic explosives by catalytic thermal dissociation blue diode laser cavity ring-down spectroscopy. Chem Phys Lett. 2013;582(0):15–20. https://doi.org/10.1016/j.cplett.2013.07.040.
Sharpe SW, Johnson TJ, Sams RL, Chu PM, Rhoderick GC, Johnson PA. Gas-phase databases for quantitative infrared spectroscopy. Appl Spectrosc. 2004;58(12):1452–61.
Furgeson A, Mielke LH, Paul D, Osthoff HD. A photochemical source of peroxypropionic and peroxyisobutanoic nitric anhydride. Atmos Environ. 2011;45(28):5025–32. https://doi.org/10.1016/j.atmosenv.2011.03.072.
Oxley JC, Smith JL, Bowden PR, Rettinger RC. Factors influencing Triacetone Triperoxide (TATP) and Diacetone Diperoxide (DADP) formation: part I. Propellants Explos Pyrotech. 2013;38(2):244–54. https://doi.org/10.1002/prep.201200116.
Roberts JM. The atmospheric chemistry of organic nitrates. Atmos Environ A. 1990;24(2):243–87. https://doi.org/10.1016/0960-1686(90)90108-Y.
Brauer B, Dubnikova F, Zeiri Y, Kosloff R, Gerber RB. Vibrational spectroscopy of triacetone triperoxide (TATP): anharmonic fundamentals, overtones and combination bands. Spectrochim Acta A Mol Biomol Spectrosc. 2008;71(4):1438–45. https://doi.org/10.1016/j.saa.2008.04.022.
Weschler CJ. Ozone in indoor environments: concentration and chemistry. Indoor Air. 2000;10(4):269–88. https://doi.org/10.1034/j.1600-0668.2000.010004269.x.
Jakobi G, Fabian P. Indoor/outdoor concentrations of ozone and peroxyacetyl nitrate (PAN). Int J Biometeorol. 1997;40(3):162–5. https://doi.org/10.1007/s004840050037.
Paul D, Furgeson A, Osthoff HD. Measurement of total alkyl and peroxy nitrates by thermal decomposition cavity ring-down spectroscopy. Rev Sci Instrum. 2009;80:114101. https://doi.org/10.1063/1.3258204.
Thaler RD, Mielke LH, Osthoff HD. Quantification of nitryl chloride at part per trillion mixing ratios by thermal dissociation cavity ring-down spectroscopy. Anal Chem. 2011;83(7):2761–6. https://doi.org/10.1021/ac200055z.
Brown SS, Stark H, Ciciora SJ, McLaughlin RJ, Ravishankara AR. Simultaneous in situ detection of atmospheric NO3 and N2O5 via cavity ring-down spectroscopy. Rev Sci Instrum. 2002;73(9):3291–301. https://doi.org/10.1063/1.1499214.
Phillips GJ, Pouvesle N, Thieser J, Schuster G, Axinte R, Fischer H, et al. Peroxyacetyl nitrate (PAN) and peroxyacetic acid (PAA) measurements by iodide chemical ionisation mass spectrometry: first analysis of results in the boreal forest and implications for the measurement of PAN fluxes. Atmos Chem Phys. 2013;13(3):1129–39. https://doi.org/10.5194/acp-13-1129-2013.
Pacheco-Londono L, Pena AJ, Primera OM, Hernandez-Rivera SP, Mina N, Garcia R, et al. An experimental and theoretical study of the synthesis and vibrational spectroscopy of triacetone triperoxide (TATP). In: Carapezza EM (ed) Sensors, and Command, Control, Communications, and Intelligence(C31) Technologies for Homeland Security and Homeland Defense Iii, Pts 1 and 2, vol 5403. Proceedings of the Society of Photo-Optical Instrumentation Engineers (Spie). Spie-Int Soc Optical Engineering, Bellingham; 2004. pp 279–287. https://doi.org/10.1117/12.542851
Hildenbrand J, Herbst J, Wollenstein J, Lambrecht A. Explosive detection using infrared laser spectroscopy. In: Razeghi M, Sudharsanan R, Brown GJ (eds) Quantum sensing and nanophotonic devices VI, vol 7222. Proceedings of SPIE. Spie-Int Soc Optical Engineering, Bellingham; 2009. p 72220B. https://doi.org/10.1117/12.808976.
Guo C, Persons J, Woodford JN, Harbison GS. Gas-phase infrared and NMR investigation of the conformers of diacetone diperoxide (DADP). J Phys Chem A. 2015;119(40):10221–8. https://doi.org/10.1021/acs.jpca.5b07074.
Kolla P. The application of analytical methods to the detection of hidden explosives and explosive devices. Angew Chem Int Ed. 1997;36(8):800–11. https://doi.org/10.1002/anie.199708001.
Rücker C, Kümmerer K. Environmental chemistry of organosiloxanes. Chem Rev. 2015;115(1):466–524. https://doi.org/10.1021/cr500319v.
Almond MJ, Becerra R, Bowes SJ, Cannady JP, Ogden JS, Walsh R. A mechanistic study of cyclic siloxane pyrolyses at low pressures. Phys Chem Chem Phys. 2008;10(45):6856–61. https://doi.org/10.1039/b812535j.
Davidson IMT, Thompson JF. Dimethylsilanone from the pyrolysis of octamethylcyclotetrasiloxane. J Chem Soc D: Chem Commun. 1971;6:251–2. https://doi.org/10.1039/c29710000251.
Kulyk K, Zettergren H, Gatchell M, Alexander JD, Borysenko M, Palianytsia B, et al. Dimethylsilanone generation from pyrolysis of polysiloxanes filled with nanosized silica and ceria/silica. ChemPlusChem. 2016;81(9):1003–13. https://doi.org/10.1002/cplu.201600229.
Acknowledgments
The authors thank Ezra Wood for sharing a preprint version of a manuscript and useful discussions.
Funding
This work was made possible by the financial support of the Natural Science and Engineering Research Council of Canada (NSERC) through a “Discovery grant.” YMT and MTS received financial support from NSERC’s Collaborative Research and Training Experience (CREATE) Program Integrating Atmospheric Chemistry and Physics from Earth to Space (IACPES).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(PDF 623 kb)
Rights and permissions
About this article
Cite this article
Taha, Y.M., Saowapon, M.T. & Osthoff, H.D. Detection of triacetone triperoxide by thermal decomposition peroxy radical chemical amplification coupled to cavity ring-down spectroscopy. Anal Bioanal Chem 410, 4203–4212 (2018). https://doi.org/10.1007/s00216-018-1072-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-018-1072-0