Abstract
Angiotensin II type 1 receptor (AT1R), a typical G protein-coupled receptor, plays a key role in regulating many cardiovascular functions. Different ligands can bind with AT1R to selectively activate either G protein (Gq) or β-arrestin (β-arr) pathway, or both pathways, but the molecular mechanism is not clear yet. In this work, we used, for the first time, atomic force microscopy-based single molecule force spectroscopy (SMFS) to study the interactions of AT1R with three types of ligands, balanced ligand, Gq-biased ligand, and β-arr-biased ligand, in living cells. The results revealed their difference in binding force and binding stability. The complex of the Gq-biased ligand-AT1R overcame two energy barriers with an intermediate state during dissociation, whereas that of β-arr-biased ligand–AT1R complex overcame one energy barrier. This indicated that AT1R had different ligand-binding conformational substates and underwent different structural changes to activate downstream signaling pathways with variable agonist efficacies. Quantitative analysis of AT1R-ligand binding in living cells at the single-molecule level offers a new tool to study the molecular mechanism of AT1R biased activation.

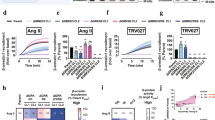
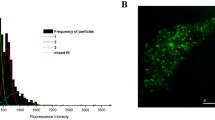
Single-molecule force measurement on the living cell expressing AT1R-eGFP with a ligand modified AFM tip (left), the dynamic force spectra of β-arrestin biased ligands-AT1R (middle), and Gq-biased ligands-AT1R (right). The complexes of β-arr-biased ligand-AT1R overcame one energy barrier, with one linear region in the spectra, whereas the Gq-biased ligand-AT1R complexes overcame two energy barriers with two linear regions




Similar content being viewed by others
References
De Gasparo M, Catt K, Inagami T, Wright J, Unger T. International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol Rev. 2000;52(3):415–72.
Balakumar P, Jagadeesh G. A century old renin–angiotensin system still grows with endless possibilities: AT 1 receptor signaling cascades in cardiovascular physiopathology. Cell Signal. 2014;26(10):2147–60.
Hunyady L, Catt KJ. Pleiotropic AT1 receptor signaling pathways mediating physiological and pathogenic actions of angiotensin II. Mol Endocrinol. 2006;20(5):953–70.
Strachan RT, Sun J-p, Rominger DH, Violin JD, Ahn S, Thomsen ARB, et al. Divergent transducer-specific molecular efficacies generate biased agonism at a G protein-coupled receptor (GPCR). J Biol Chem. 2014;289(20):14211–24.
Rajagopal S, Ahn S, Rominger DH, Gowen-MacDonald W, Lam CM, DeWire SM, et al. Quantifying ligand bias at seven-transmembrane receptors. Mol Pharmacol. 2011;80(3):367–77.
Revankar CM, Vines CM, Cimino DF, Prossnitz ER. Arrestins block G protein-coupled receptor-mediated apoptosis. J Biol Chem. 2004;279(23):24578–84.
Whalen EJ, Rajagopal S, Lefkowitz RJ. Therapeutic potential of β-arrestin- and G protein-biased agonists. Trends Mol Med. 2011;17(3):126–39.
Paradis P, Dali-Youcef N, Paradis FW, Thibault G, Nemer M. Overexpression of angiotensin II type I receptor in cardiomyocytes induces cardiac hypertrophy and remodeling. Proc Natl Acad Sci U S A. 2000;97(2):931–6.
Wisler JW, Xiao K, Thomsen AR, Lefkowitz RJ. Recent developments in biased agonism. Curr Opin Cell Biol. 2014;27:18–24.
Monasky MM, Taglieri DM, Henze M, Warren CM, Utter MS, Soergel DG, et al. The β-arrestin-biased ligand TRV120023 inhibits angiotensin II-induced cardiac hypertrophy while preserving enhanced myofilament response to calcium. Am J Physiol Heart Circ Physiol. 2013;305(6):H856–66.
Violin JD, Soergel DG, Boerrigter G, Burnett JC, Lark MW. GPCR biased ligands as novel heart failure therapeutics. Trends Cardiovasc Med. 2013;23(7):242–9.
DeWire SM, Violin JD. Biased ligands for better cardiovascular drugs. Circ Res. 2011;109(2):205–16.
Kim KS, Abraham D, Williams B, Violin JD, Mao L, Rockman HA. β-Arrestin-biased AT1R stimulation promotes cell survival during acute cardiac injury. Am J Physiol Heart Circ Physiol. 2012;303(8):H1001–10.
Zhang H, Unal H, Desnoyer R, Han GW, Patel N, Katritch V, et al. Structural basis for ligand recognition and functional selectivity at angiotensin receptor. J Biol Chem. 2015;290(49):29127–39.
Unal H, Jagannathan R, Bhatnagar A, Tirupula K, Desnoyer R, Karnik SS. Long range effect of mutations on specific conformational changes in the extracellular loop 2 of angiotensin II type 1 receptor. J Biol Chem. 2013;288(1):540–51.
Cabana J, Holleran B, Leduc R, Escher E, Guillemette G, Lavigne P. Identification of distinct conformations of the angiotensin-II type 1 receptor associated with the Gq/11 protein pathway and the β-arrestin pathway using molecular dynamics simulations. J Biol Chem. 2015;290(25):15835–54.
Kenakin T, Miller LJ. Seven transmembrane receptors as shapeshifting proteins: the impact of allosteric modulation and functional selectivity on new drug discovery. Pharmacol Rev. 2010;62(2):265–304.
Rahmeh R, Damian M, Cottet M, Orcel H, Mendre C, Durroux T, et al. Structural insights into biased G protein-coupled receptor signaling revealed by fluorescence spectroscopy. Proc Natl Acad Sci U S A. 2012;109(17):6733–8.
Sridharan R, Zuber J, Connelly SM, Mathew E, Dumont ME. Fluorescent approaches for understanding interactions of ligands with G protein coupled receptors. Biochim Biophys Acta Biomembr. 2014;1838(1):15–33.
Devost D, Sleno R, Pétrin D, Zhang A, Shinjo Y, Okde R, et al. Conformational profiling of the AT1 angiotensin II receptor reflects biased agonism, G protein coupling, and cellular context. J Biol Chem. 2017;292(13):5443–56.
Müller DJ, Dufrene YF. Atomic force microscopy as a multifunctional molecular toolbox in nanobiotechnology. Nat Nanotechnol. 2008;3(5):261–9.
Hinterdorfer P, Dufrêne YF. Detection and localization of single molecular recognition events using atomic force microscopy. Nat Methods. 2006;3(5):347–55.
Butt HJ, Cappella B, Kappl M. Force measurements with the atomic force microscope: technique, interpretation, and applications. Surf Sci Rep. 2005;59(1):1–152.
Yang Y, Xu Y, Xia T, Chen F, Zhang C, Liang W, et al. A single-molecule study of the inhibition effect of Naringenin on transforming growth factor-β ligand–receptor binding. Chem Commun. 2011;47(19):5440–2.
Jiang Y, Zhu C, Ling L, Wan L, Fang X, Bai C. Specific aptamer−protein interaction studied by atomic force microscopy. Anal Chem. 2003;75(9):2112–6.
O’Donoghue MB, Shi X, Fang X, Tan W. Single-molecule atomic force microscopy on live cells compares aptamer and antibody rupture forces. Anal Bioanal Chem. 2012;402(10):3205–9.
Liu J, Zhang X, Yang H, Yuan J, Wei H, Yu J, et al. Study of the interactions between endolysin and bacterial peptidoglycan on S. aureus by dynamic force spectroscopy. Nanoscale. 2015;7(37):15245–50.
Pi J, Cai H, Yang F, Jin H, Liu J, Yang P, et al. Atomic force microscopy based investigations of anti-inflammatory effects in lipopolysaccharide-stimulated macrophages. Anal Bioanal Chem. 2016;408(1):165–76.
Cuerrier CM, Benoit M, Guillemette G, Grandbois M. Real-time monitoring of angiotensin II-induced contractile response and cytoskeleton remodeling in individual cells by atomic force microscopy. Pflugers Arch. 2009;457(6):1361–72.
Hong K, Zhao G, Hong Z, Sun Z, Yang Y, Clifford PS, et al. Mechanical activation of angiotensin II type 1 receptors causes actin remodelling and myogenic responsiveness in skeletal muscle arterioles. J Physiol. 2016;594(23):7027–47.
Jeong KH, Lee TW, Ihm CG, Moon JY, Lee GJ, Park KH, et al. Real-time monitoring of the effects of telmisartan on angiotensin II-induced mechanical changes in live mesangial cells using atomic force microscopy. Kidney Blood Press Res. 2012;35(6):573–82.
Lee GJ, Park EJ, Choi S, Park JH, Jeong KH, Kim KS, et al. Observation of angiotensin II-induced changes in fixed and live mesangial cells by atomic force microscopy. Micron. 2010;41(3):220–6.
Simard E, Kovacs JJ, Miller WE, Kim J, Grandbois M, Lefkowitz RJ. β-Arrestin regulation of myosin light chain phosphorylation promotes AT1aR-mediated cell contraction and migration. PLoS One. 2013;8(11):e80532.
Zhang X, Shi X, Xu L, Yuan J, Fang X. Atomic force microscopy study of the effect of HER 2 antibody on EGF mediated ErbB ligand–receptor interaction. Nanomedicine. 2013;9(5):627–35.
Hein L, Meinel L, Pratt RE, Dzau VJ, Kobilka BK. Intracellular trafficking of angiotensin II and its AT1 and AT2 receptors: evidence for selective sorting of receptor and ligand. Mol Endocrinol. 1997;11(9):1266–77.
Thundat T, Zheng XY, Sharp S, Allison D, Warmack R, Joy D, et al. Calibration of atomic force microscope tips using biomolecules. Scanning Microsc. 1992;6(4):903–10.
Hutter JL, Bechhoefer J. Calibration of atomic-force microscope tips. Rev Sci Instrum. 1993;64(7):1868–73.
Merkel R, Nassoy P, Leung A, Ritchie K, Evans E. Energy landscapes of receptor–ligand bonds explored with dynamic force spectroscopy. Nature. 1999;397(6714):50–3.
Bell GI. Models for the specific adhesion of cells to cells. Science. 1978;200(4342):618–27.
Bieri O, Wirz J, Hellrung B, Schutkowski M, Drewello M, Kiefhaber T. The speed limit for protein folding measured by triplet–triplet energy transfer. Proc Natl Acad Sci U S A. 1999;96(17):9597–601.
Ge L, Jin G, Fang X. Investigation of the interaction between a bivalent aptamer and thrombin by AFM. Langmuir. 2011;28(1):707–13.
Landas S, Phillips M, Stamler J, Raizada M. Visualization of specific angiotensin II binding sites in the brain by fluorescent microscopy. Science. 1980;210(4471):791–3.
Shenoy SK, Lefkowitz RJ. Angiotensin II-stimulated signaling through G proteins and β-arrestin. Sci Signal. 2005;2005(311):cm14.
Violin JD, DeWire SM, Yamashita D, Rominger DH, Nguyen L, Schiller K, et al. Selectively engaging β-arrestins at the angiotensin II type 1 receptor reduces blood pressure and increases cardiac performance. J Pharmacol Exp Ther. 2010;335(3):572–9.
Yu J, Jiang Y, Ma X, Lin Y, Fang X. Energy landscape of aptamer/protein complexes studied by single-molecule force spectroscopy. Chem Asian J. 2007;2(2):284–9.
Zocher M, Fung JJ, Kobilka BK, Muller DJ. Ligand-specific interactions modulate kinetic, energetic, and mechanical properties of the human beta2 adrenergic receptor. Structure. 2012;20(8):1391–402.
Shi X, Xu L, Yu J, Fang X. Study of inhibition effect of Herceptin on interaction between Heregulin and ErbB receptors HER3/HER2 by single-molecule force spectroscopy. Exp Cell Res. 2009;315(16):2847–55.
Alsteens D, Pfreundschuh M, Zhang C, Spoerri PM, Coughlin SR, Kobilka BK, et al. Imaging G protein-coupled receptors while quantifying their ligand-binding free-energy landscape. Nat Methods. 2015;12(9):845–51.
Bustanji Y, Arciola CR, Conti M, Mandello E, Montanaro L, Samorí B. Dynamics of the interaction between a fibronectin molecule and a living bacterium under mechanical force. Proc Natl Acad Sci U S A. 2003;100(23):13292–7.
Acknowledgments
This work was supported by National Basic Research Program of China (2013CB933701), National Natural Science Foundation of China (no. 21735006, 21127901, 91413119), and Chinese Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Published in the topical collection Euroanalysis XIX with guest editors Charlotta Turner and Jonas Bergquist.
Rights and permissions
About this article
Cite this article
Li, W., Xu, J., Kou, X. et al. Single-molecule force spectroscopy study of interactions between angiotensin II type 1 receptor and different biased ligands in living cells. Anal Bioanal Chem 410, 3275–3284 (2018). https://doi.org/10.1007/s00216-018-0956-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-018-0956-3