Abstract
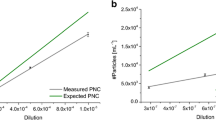
Single particle ICP-MS has evolved rapidly as a quantitative method for determining nanoparticle size and number concentration at environmentally relevant exposure levels. Central to the application of spICP-MS is a commonly used, but not rigorously validated, calibration approach based on the measured transport efficiency and the response of ionic standards. In this work, we present a comprehensive and systematic study of the accuracy, precision and robustness of spICP-MS using the rigorously characterized reference material (RM) 8017 (Polyvinylpyrrolidone Coated Nominal 75 nm Silver Nanoparticles), recently issued by the National Institute of Standards and Technology (NIST). We report for the first time, statistically significant differences in frequency-based and size-based measures of transport efficiency with NIST RM 8013 Gold Nanoparticles and demonstrate that the size-based measure of transport efficiency is more robust and yields accurate results for the silver nanoparticle RM relative to TEM-based reference values. This finding is significant, because the frequency-based method is more widely applied. Furthermore, we demonstrate that the use of acidified ionic standards improves measurement of ICP-MS Ag response, but does not degrade the accuracy of the results for AgNP suspensions in water or various other diluents. Approaches for controlling AgNP dissolution were investigated and are shown to effectively improve particle stability in dilute suspensions required for spICP-MS analysis, while minimally affecting the measured intensity and allowing for more robust analysis. This study is an important and necessary advancement toward full validation and adoption of spICP-MS by the broader research community.

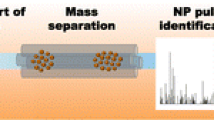
Measurement challenges in spICP-MS analysis






Similar content being viewed by others
References
Vance ME, Kuiken T, Vejerano EP, McGinnis SP, Hochella MF, Rejeski D, et al. Nanotechnology in the real world: redeveloping the nanomaterial consumer products inventory. Beilstein J Nanotechnol. 2015;6:1769–80.
Wiesner MR, Lowry GV, Alvarez P, Dionysiou D, Biswas P. Assessing the risks of manufactured nanomaterials. Environ Sci Technol. 2006;40:4336–45.
Ju-Nam Y, Lead JR. Manufactured nanoparticles: an overview of their chemistry, interactions and potential environmental implications. Sci Total Environ. 2008;400:396–414.
Colvin VL. The potential environmental impact of engineered nanomaterials. Nat Biotechnol. 2003;21:1166–70.
Mitrano DM, Motellier S, Clavaguera S, Nowack B. Review of nanomaterial aging and transformations through the life cycle of nano-enhanced products. Environ Int. 2015;77:132–47.
Laborda F, Bolea E, Cepria G, Gomez MT, Jimenez MS, Perez-Arantegui J, et al. Detection, characterization and quantification of inorganic engineered nanomaterials: a review of techniques and methodological approaches for the analysis of complex samples. Anal Chim Acta. 2016;904:10–32.
Laborda F, Bolea E, Jimenez-Lamana J. Single particle inductively coupled plasma mass spectrometry: a powerful tool for nanoanalysis. Anal Chem. 2014;86:2270–8.
Degueldre C, Favarger PY. Colloid analysis by single particle inductively coupled plasma-mass spectroscopy: a feasibility study. Colloid Surface A. 2003;217:137–42.
Tan J, Liu J, Li M, Hadri HE, Hackley VA, Zechariah MR. Electrospray-differential mobility hyphenated with single particle inductively coupled plasma mass spectrometry for characterization of nanoparticles and their aggregates. Anal Chem. 2016;88:8548–55.
Laborda F, Jimenez-Lamana J, Bolea E, Castillo JR. Critical considerations for the determination of nanoparticle number concentrations, size and number size distributions by single particle ICP-MS. J Anal Atom Spectrom. 2013;28:1220–32.
Tuoriniemi J, Cornelis G, Hassellov M. Size discrimination and detection capabilities of single-particle ICPMS for environmental analysis of silver nanoparticles. Anal Chem. 2012;84:3965–72.
Olesik JW, Gray PJ. Considerations for measurement of individual nanoparticles or microparticles by ICP-MS: determination of the number of particles and the analyte mass in each particle. J Anal Atom Spectrom. 2012;27:1143–55.
Ho KS, Lui KO, Lee KH, Chan WT. Considerations of particle vaporization and analyte diffusion in single-particle inductively coupled plasma-mass spectrometry. Spectrochim Acta B. 2013;89:30–9.
Lee WW, Chan WT. Calibration of single-particle inductively coupled plasma-mass spectrometry (SP-ICP-MS). J Anal Atom Spectrom. 2015;30:1245–54.
Strenge I, Engelhard C. Capabilities of fast data acquisition with microsecond time resolution in inductively coupled plasma mass spectrometry and identification of signal artifacts from millisecond dwell times during detection of single gold nanoparticles. J Anal Atom Spectrom. 2016;31:135–44.
Liu J, Murphy KE, MacCuspie RI, Winchester MR. Capabilities of single particle inductively coupled plasma mass spectrometry for the size measurement of nanoparticles: a case study on gold nanoparticles. Anal Chem. 2014;86:3405–14.
Peters RJB, Rivera ZH, van Bemmel G, Marvin HJP, Weigel S, Bouwmeester H. Development and validation of single particle ICP-MS for sizing and quantitative determination of nano-silver in chicken meat. Anal Bioanal Chem. 2014;406:3875–85.
Peters R, Herrera-Rivera Z, Undas A, van der Lee M, Marvin H, Bouwmeester H, et al. Single particle ICP-MS combined with a data evaluation tool as a routine technique for the analysis of nanoparticles in complex matrices. J Anal Atom Spectrom. 2015;30:1274–85.
Cornelis G, Hassellov M. A signal deconvolution method to discriminate smaller nanoparticles in single particle ICP-MS. J Anal Atom Spectrom. 2014;29:134–44.
Pace HE, Rogers NJ, Jarolimek C, Coleman VA, Higgins CP, Ranville JF. Determining transport rfficiency for the purpose of counting and sizing nanoparticles via single particle inductively coupled plasma mass spectrometry. Anal Chem. 2011;83:9361–9.
Telgmann L, Metcalfe CD, Hintelmann H. Rapid size characterization of silver nanoparticles by single particle ICP-MS and isotope dilution. J Anal Atom Spectrom. 2014;29:1265–72.
Tuoriniemi J, Cornelis G, Hassellov M. Improving the accuracy of single particle ICPMS for measurement of size distributions and number concentrations of nanoparticles by determining analyte partitioning during nebulization. J Anal Atom Spectrom. 2014;29:743–52.
Hadri HE, Petersen EJ, Winchester MR. Impact of and correction for instrument sensitivity drift on nanoparticle size measurements by single-particle ICP-MS. Anal Bioanal Chem. 2016;408:5099–108.
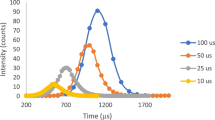
Montano MD, Badiei HR, Bazargan S, Ranville JF. Improvements in the detection and characterization of engineered nanoparticles using spICP-MS with microsecond dwell times. Environ Sci Nano. 2014;1:338–46.
Abad-Alvaro I, Pena-Vazquez E, Bolea E, Bermejo-Barrera P, Castillo JR, Laborda F. Evaluation of number concentration quantification by single-particle inductively coupled plasma mass spectrometry: microsecond vs. millisecond dwell times. Anal Bioanal Chem. 2016;408:5089–97.
Bustos ARM, Petersen EJ, Possolo A, Winchester MR. Post hoc interlaboratory comparison of single particle ICP-MS size measurements of NIST gold nanoparticle reference materials. Anal Chem. 2015;87:8809–17.
Montano MD, Olesik JW, Barber AG, Challis K, Ranville JF. Single particle ICP-MS: advances toward routine analysis of nanomaterials. Anal Bioanal Chem. 2016;408:5053–74.
Donovan AR, Adams CD, Ma Y, Stephan C, Eichholz T, Shi H. Single particle ICP-MS characterization of titanium dioxide, silver, and gold nanoparticles during drinking water treatment. Chemosphere. 2016;144:148–53.
Loeschner K, Navratilova J, Kobler C, Molhave K, Wagner S, von der Kammer F, et al. Detection and characterization of silver nanoparticles in chicken meat by asymmetric flow field flow fractionation with detection by conventional or single particle ICP-MS. Anal Bioanal Chem. 2016;405:8185–95.
Gray EP, Coleman JG, Bednar AJ, Kennedy AJ, Ranville JF, Higgins CP. Extraction and analysis of silver and gold nanoparticles from biological tissues using single particle inductively coupled plasma mass spectrometry. Environ Sci Technol. 2013;47:14315–23.
Mitrano DM, Ranville JF, Bednar A, Kazor K, Hering AS, Higgins CP. Tracking dissolution of silver nanoparticles at environmentally relevant concentrations in laboratory, natural, and processed waters using single particle ICP-MS (spICP-MS). Environ Sci Nano. 2014;1:248–59.
Ramos K, Gomez-Gomez MM, Camara C, Ramos L. Silver speciation and characterization of nanoparticles released from plastic food containers by single particle ICPMS. Talanta. 2016;151:83–90.
Murphy KE, Liu J, Guthrie WF, Gorham JM, Bonevich JE, Allen AJ, Winchester MR, Hackley VA, MacCuspie RI. Use of single particle inductively coupled plasma mass spectrometry to characterize a new silver nanoparticle reference material. Nanotechnology 2014: Graphene, CNTs, Particles, Films & Composites Technical Proceedings of the 2014 NSTI Nanotechnolgy Conference and Expo, CRC Press. 2014; pp. 501–4.
Linsinger TPJ, Peters R, Weigel S. International interlaboratory study for sizing and quantification of ag nanoparticles in food simulants by single-particle ICPMS. Anal Bioanal Chem. 2014;406:3835–43.
Navratilova J, Praetorius A, Gondikas A, Fabienke W, von der Kammer F, Hofmann T. Detection of engineered copper nanoparticles in soil using single particle ICP-MS. Int J Environ Res Public Health. 2015;12:15756–68.
Hadioui M, Merdzan V, Wilkinson KJ. Detection and characterization of ZnO nanoparticles in surface and waste waters using single particle ICPMS. Environ Sci Technol. 2015;49:6141–8.
Donovan AR, Adams CD, Ma Y, Stephan C, Eichholz T, Shi H. Detection of zinc oxide and cerium dioxide nanoparticles during drinking water treatment by rapid single particle ICP-MS methods. Anal Bioanal Chem. 2016;408:5137–45.
Frechette-Viens L, Hadioui M, Wilkinson KJ. Practical limitations of single particle ICP-MS in the determination of nanoparticle size distributions and dissolution: case of rare earth oxides. Talanta. 2017;163:121–6.
Pace HE, Rogers NJ, Jarolimek C, Coleman VA, Gray EP, Higgins CP, et al. Single particle inductively coupled plasma-mass spectrometry: a performance evaluation and method comparison in the determination of nanoparticle size. Environ Sci Technol. 2012;46:12272–80.
Murphy KE, Liu J, Bustos ARM, Johnson ME, Winchester MR. Characterization of nanoparticle suspensions using single particle inductively coupled plasma mass spectrometry. NIST Special Publication-1200-2. 2015. doi:10.6028/NIST.SP.1200-21.
Spreadsheet Single Particle Calculation Tool. In: Single Particle Calculation tool. Wageningen University & Research. 2017. https://www.wur.nl/en/show/Single-Particle-Calculation-tool.htm. Accessed 28 June 2017.
Report of Investigation for Reference Material 8017, Polyvinylpyrrolidone Coated Silver Nanoparticles, Nominal Diameter 75 nm. National Institute of Standards and Technology. Gaithersburg, MD, 2015.
Liu J, Hurt RH. Ion release kinetics and particle persistence in aqueous nano-silver colloids. Environ Sci Technol. 2010;44:2169–75.
Methods for measuring the acute toxicity of effluents and receiving waters to freshwater and marine organisms. EPA-821-R-02-012. U.S. Environmental Protection Agency, Washington, DC 20460, 2002.
Sekine R, Khurana K, Vasilev K, Lombi E, Donner E. Quantifying the adsorption of ionic silver and functionalized nanoparticles during ecotoxicity testing: test container effects and recommendations. Nanotoxicology. 2015;9:1005–12.
Chen W, Wee P, Brindle ID. Elimination of the memory effects of gold, mercury and silver in inductively coupled plasma atomic emission spectroscopy. J Anal Atom Spectrom. 2000;15:409–13.
Ramkorun-Schmidt B, Pergantis SA, Esteban-Fernandez D, Jakubowski N, Gunther D. Investigation of a combined microdroplet generator and pneumatic nebulization system for quantitative determination of metal-containing nanoparticles using ICPMS. Anal Chem. 2015;87:8687–94.
Gschwind S, Hagendorfer H, Frick DA, Gunther D. Mass quantification of nanoparticles by single droplet calibration using inductively coupled plasma mass spectrometry. Anal Chem. 2013;85:5875–83.
Garcia CC, Murtazin A, Groh S, Horvatic V, Niemax K. Characterization of single au and SiO2 nano- and microparticles by ICP-OES using monodisperse droplets of standard solutions for calibration. J Anal Atom Spectrom. 2010;25:645–53.
Laborda F, Jimenez-Lamana J, Bolea E, Castillo JR. Selective identification, characterization and determination of dissolved silver (I) and silver nanoparticles based on single particle detection by inductively coupled plasma mass spectrometry. J Anal Atom Spectrom. 2011;26:1362–71.
Kittler S, Greulich C, Diendorf J, Koller M, Epple M. Toxicity of silver nanoparticles increases during storage because of slow dissolution under release of silver ions. Chem Mater. 2010;22:4548–54.
Liu J, Sonshine DA, Shervani S, Hurt RH. Controlled release of biologically active silver from nanosilver surfaces. ACS Nano. 2010;4:6903–13.
Gorham JM, Rohlfing AB, Lippa KA, MacCuspie RI, Hemmati A, Holbrook RD. Storage wars: how citrate-capped silver nanoparticle suspensions are affected by not-so-trivial decisions. J Nanopart Res. 2014;16:2339–53.
Liu J, Pennell KG, Hurt RH. Kinetics and mechanisms of nanosilver oxysulfidation. Environ Sci Technol. 2011;45:7345–53.
Levard C, Reinsch BC, Michel FM, Oumahi C, Lowry GV, Brown GE. Sulfidation processes of PVP-coated silver nanoparticles in aqueous solution: impact on dissolution rate. Environ Sci Technol. 2011;45:5260–6.
Thalmann B, Voegelin A, Sinnet B, Morgenroth E, Kaegi R. Sulfidation kinetics of silver nanoparticles reacted with metal sulfides. Environ Sci Technol. 2014;48:4885–92.
Kent RD, Oser JG, Vikesland PJ. Controlled evaluation of silver nanoparticle sulfidation in a full-scale wastewater treatment plant. Environ Sci Technol. 2014;48:8564–72.
Pettibone JM, Liu J. In situ methods for monitoring silver nanoparticle sulfidation in simulated waters. Environ Sci Technol. 2016;50:11145–53.
Schmidt M, Masson A, Brechignac C. Oxygen and silver clusters: transition from chemisorption to oxidation. Phys Rev Lett. 2003;91:243401.
Sotebier CA, Kutscher DJ, Rottmann L, Jakubowski N, Panne U, Bettmer J. Combination of single particle ICP-QMS and isotope dilution analysis for the determination of size, particle number and number size distribution of silver nanoparticles. J Anal Atom Spectrom. 2016;31:2045–52.
Acknowledgements
The authors would like to thank Arnab K. Mukherjee (Materials Measurement Science Division, NIST), Antonio R. Montoro Bustos and Lee L. Yu (Chemical Sciences Division, NIST) for their thorough reviews of the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest
Electronic supplementary material
ESM 1
(PDF 1043 kb)
Rights and permissions
About this article
Cite this article
Liu, J., Murphy, K.E., Winchester, M.R. et al. Overcoming challenges in single particle inductively coupled plasma mass spectrometry measurement of silver nanoparticles. Anal Bioanal Chem 409, 6027–6039 (2017). https://doi.org/10.1007/s00216-017-0530-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-017-0530-4