Abstract
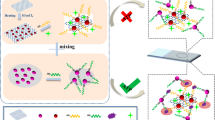
A supramolecular recognition and oriented assembly system was developed on chip for the highly selective surface-enhanced Raman scattering (SERS) detection of thrombin by means of the aptamer-based SERS tag method. A 15-base thrombin-binding aptamer (TBA15) with a thiol end was first immobilized on an Ag nanoprism array by the S–Ag bond. This aptamer has high binding affinity with thrombin when it folds into a G-quadruplex structure. After the recognition between the aptamer and thrombin, a bridge is built between the SERS tag (4-mercaptobenzoic acid marked Ag nanoparticle) and the fixed thrombin based on the activation of the carboxylic group of 4-mercaptobenzoic acid. Thus, the quantitative detection of thrombin can be achieved based on the SERS intensity of the immobilized SERS tags. The obvious advantages of this sensing method are as follows: (1) remarkable SERS enhancement due to the high electric field coupling effect via the gap structure formation, which improves the sensitivity of the SERS detection and the limit of detection of this method arrives in 1.6 × 10–11 M, (2) high selectivity based on the specific aptamer recognition toward thrombin, which can be extended to other enzymes easily by changing a proper sequence, (3) high repeatability of SERS signals according to a highly ordered structure, and (4) highly efficient oriented assembly of a sandwich structure over an Ag nanoprism array. The proposed method is expected to be a practical implement in medical diagnosis.

Illustration of the aptamer-based SERS sensor for thrombin detection






Similar content being viewed by others
References
Yoon J, Choi N, Ko J, Kim K, Lee S, Choo J. Highly sensitive detection of thrombin using SERS-based magnetic aptasensors. Biosens Bioelectron. 2013;47:62–7.
Rahman MA, Son JI, Won MS, Shim YB. Gold nanoparticles doped conducting polymer nanorod electrodes: ferrocene catalyzed aptamer-based thrombin immunosensor. Anal Chem. 2009;81:6604–11.
Du F, Alam MN, Pawliszyn J. Aptamer-functionalized solid phase microextraction-liquid chromatography/tandem mass spectrometry for selective enrichment and determination of thrombin. Anal Chim Acta. 2014;845:45–52.
Zhao Q, Wang X. An aptamer-capture based chromogenic assay for thrombin. Biosens Bioelectron. 2012;34:232–7.
Gao F, Du L, Tang D, Lu Y, Zhang Y, Zhang L. A cascade signal amplification strategy for surface enhanced Raman spectroscopy detection of thrombin based on DNAzyme assistant DNA recycling and rolling circle amplification. Biosens Bioelectron. 2015;66:423–30.
Centi S, Tombelli S, Minunni M, Mascini M. Aptamer-based detection of plasma proteins by an electrochemical assay coupled to magnetic beads. Anal Chem. 2007;79:1466–73.
Zhao J, Zhang Y, Li H, Wen Y, Fan X, Lin F, et al. Ultrasensitive electrochemical aptasensor for thrombin based on the amplification of aptamer-AuNPs-HRP conjugates. Biosens Bioelectron. 2011;26:2297–303.
Mani RJ, Dye RG, Snider TA, Wang S, Clinkenbeard KD. Bi-cell surface plasmon resonance detection of aptamer mediated thrombin capture in serum. Biosens Bioelectron. 2011;26:4832–6.
Zuo X, Song S, Zhang J, Pan D, Wang L, Fan C. A target-responsive electrochemical aptamer switch (TREAS) for reagentless detection of nanomolar ATP. J Am Chem Soc. 2007;129:1042–3.
Yu J, Yang L, Liang X, Dong T, Liu H. Bare magnetic nanoparticles as fluorescence quenchers for detection of thrombin. Analyst. 2015;140:4114–20.
Xue Q, Liu Z, Guo Y, Guo S. Cyclodextrin functionalized graphene-gold nanoparticle hybrids with strong supramolecular capability for electrochemical thrombin aptasensor. Biosens Bioelectron. 2015;68:429–36.
Lv Z, Liu J, Bai W, Yang S, Chen A. A simple and sensitive label-free fluorescent approach for protein detection based on a perylene probe and aptamer. Biosens Bioelectron. 2015;64:530–4.
Stiles PL, Dieringer JA, Shah NC, Van Duyne RR. Surface-enhanced Raman spectroscopy. Annu Rev Anal Chem. 2008;1:601–26.
Qian XM, Nie SM. Single-molecule and single-nanoparticle SERS: from fundamental mechanisms to biomedical applications. Chem Soc Rev. 2008;37:912–20.
Fu C, Xu W, Wang H, Ding H, Liang L, Cong M, et al. DNAzyme-based plasmonic nanomachine for ultrasensitive selective surface-enhanced Raman scattering detection of lead ions via a particle-on-a-film hot spot construction. Anal Chem. 2014;86:11494–7.
Chen JH, Li YY, Huang K, Wang PX, He LL, Carter KR. Nanoimprinted patterned pillar substrates for surface-enhanced Raman scattering applications. ACS Appl Mater Interfaces. 2015;7:22106–13.
Lim DK, Jeon KS, Kim HM, Nam JM, Suh YD. Nanogap-engineerable Raman-active nanodumbbells for single-molecule detection. Nat Mater. 2010;9:60–7.
Nie SM. Probing single molecules and single nanoparticles by surface-enhanced Raman scattering. Science. 1997;2275:1102–11.
Guo H, Xu W, Zhou J, Xu S, Lombardi JR. Highly efficient construction of oriented sandwich structures for surface-enhanced Raman scattering. Nanotechnology. 2013;24:045608 (8pp).
Balamurugan S, Obubuafo A, Soper SA, Spivak DA. Surface immobilization methods for aptamer diagnostic applications. Anal Bioanal Chem. 2008;390:1009–21.
Iliuk AB, Hu L, Tao WA. Aptamer in bioanalytical applications. Anal Chem. 2011;83:4440–52.
Shiang YC, Huang CC, Wang TH, Chien CW, Chang HT. Aptamer-conjugated nanoparticles efficiently control the activity of thrombin. Adv Funct Mater. 2010;20:3175–82.
Wang G, Wang Y, Chen L, Choo J. Nanomaterial-assisted aptamers for optical sensing. Biosens Bioelectron. 2010;25:1859–68.
Lu LM, Zhang XB, Kong RM, Yang B, Tan WH. A ligation-triggered DNAzyme cascade for amplified fluorescence detection of biological small molecules with zero-background signal. J Am Chem Soc. 2011;133:11686–91.
Wang H, Kim Y, Liu H, Zhu Z, Bamrungsap S, Tan W. Engineering a unimolecular DNA-catalytic probe for single lead ion monitoring. J Am Chem Soc. 2009;131:8221–6.
Zheng J, Jiao A, Yang R, Li H, Li J, Shi M, et al. Fabricating a reversible and regenerable Raman-active substrate with a biomolecule-controlled DNA nanomachine. J Am Chem Soc. 2012;134:19957–60.
Fu C, Wang Y, Chen G, Yang L, Xu S, Xu W. Aptamer-based surface-enhanced Raman scattering-microfluidic sensor for sensitive and selective polychlorinated biphenyls detection. Anal Chem. 2015;87:9555–8.
Chung E, Gao R, Ko J, Choi N, Lim DW, Lee EK, et al. Trace analysis of mercury(II) ions using aptamer-modified Au/Ag core-shell nanoparticles and SERS spectroscopy in a microdroplet channel. Lab Chip. 2013;13:260–6.
Lee P, Meisel D. Adsorption and surface-enhanced Raman of dyes on silver and gold sols. J Phys Chem. 1982;86:3391–5.
Zhou J, An J, Tang B, Xu S, Cao Y, Zhao B, et al. Growth of tetrahedral silver nanocrystals in aqueous solution and their SERS enhancement. Langmuir. 2008;24:10407–13.
Ma RZ, Sasaki T, Bando Y. Layer-by-layer assembled multilayer films of titanate nanotubes, Ag- or Au-loaded nanotubes, and nanotubes/nanosheets with polycations. J Am Chem Soc. 2004;126:10382–8.
Haynes CL, Van Duyne RP. Nanosphere lithography: a versatile nanofabrication tool for studies of size-dependent nanoparticle optics. J Phys Chem B. 2001;105:5599–611.
Jensen TR, Malinsky MD, Haynes CL, Van Duyne RP. Nanosphere lithography: tunable localized surface plasmon resonance spectra of silver nanoparticles. J Phys Chem B. 2000;104:10549–56.
Willets KA, Van Duyne RP. Localized surface plasmon resonance spectroscopy and sensing. Annu Rev Phys Chem. 2007;58:267–97.
Leng WN, Woo HY, Vak D, Bazan GC, Kelley AM. Surface-enhanced resonance Raman and hyper-Raman spectroscopy of water-soluble substituted stilbene and distyrylbenzene chromophores. J Raman Spectrosc. 2006;37:132–41.
Kukushkin VI, Van'kov AB, Kukushkin IV. Relationship between the giant enhancement of the Raman scattering and luminescence on nanostructured metallic surfaces. JETP Lett. 2013;98:342–7.
Acknowledgments
This work was supported by the National Instrumentation Program (NIP) of the Ministry of Science and Technology of China no. 2011YQ03012408 and National Natural Science Foundation of China (21573092, 21573087, and 21373096).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Concerning the content of this paper there is no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 832 kb)
Rights and permissions
About this article
Cite this article
Yang, L., Fu, C., Wang, H. et al. Aptamer-based surface-enhanced Raman scattering (SERS) sensor for thrombin based on supramolecular recognition, oriented assembly, and local field coupling. Anal Bioanal Chem 409, 235–242 (2017). https://doi.org/10.1007/s00216-016-9992-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-016-9992-z