Abstract
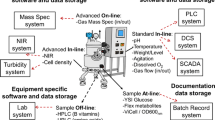
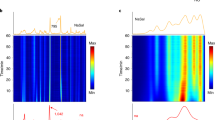
Significant improvements can be realized by converting conventional batch processes into continuous ones. The main drivers include reduction of cost and waste, increased safety, and simpler scale-up and tech transfer activities. Re-designing the process layout offers the opportunity to incorporate a set of process analytical technologies (PAT) embraced in the Quality-by-Design (QbD) framework. These tools are used for process state estimation, providing enhanced understanding of the underlying variability in the process impacting quality and yield. This work describes a road map for identifying the best technology to speed-up the development of continuous processes while providing the basis for developing analytical methods for monitoring and controlling the continuous full-scale reaction. The suitability of in-line Raman, FT-infrared (FT-IR), and near-infrared (NIR) spectroscopy for real-time process monitoring was investigated in the production of 1-bromo-2-iodobenzene. The synthesis consists of three consecutive reaction steps including the formation of an unstable diazonium salt intermediate, which is critical to secure high yield and avoid formation of by-products. All spectroscopic methods were able to capture critical information related to the accumulation of the intermediate with very similar accuracy. NIR spectroscopy proved to be satisfactory in terms of performance, ease of installation, full-scale transferability, and stability to very adverse process conditions. As such, in-line NIR was selected to monitor the continuous full-scale production. The quantitative method was developed against theoretical concentration values of the intermediate since representative sampling for off-line reference analysis cannot be achieved. The rapid and reliable analytical system allowed the following: speeding up the design of the continuous process and a better understanding of the manufacturing requirements to ensure optimal yield and avoid unreacted raw materials and by-products in the continuous reactor effluent.

Using PAT to accelerate the transition to continuous API manufacturing









Similar content being viewed by others
References
Baines D. Problems facing the pharmaceutical industry and approaches to ensure long term viability. Sch Commons. 2010;5(4):1–59.
Xiang Y, Lucas J, Van Alsten J, Li B, Preston B, Lovdahl M, et al. Using process analytical technology (PAT) tools to support flow chemistry development and production. Am Pharm Rev. 2012;15(3):1–10.
FDA. Pharmaceutical CGMPs for the 21st century—a risk-based approach. 2004.
Poechlauer P, Manley J, Broxterman R, Gregertsen B, Ridemark M. Continuous processing in the manufacture of active pharmaceutical ingredients and finished dosage forms: an industry perspective. Org Process Res Dev. 2012;16(10):1586–90.
Chen R, Bowles D, Antosz F, Xiang Y, Li S, Barrila M, Coutant M. Practical Approaches in Applying Process Analytical Technology (PAT) Tools to Early Active Pharmaceutical Ingredient (API) Development. In: Pharmaceutical Outsourcing. 2011. http://www.pharmoutsourcing.com/Featured-Articles/37503-Practical-Approaches-in-Applying-Process-Analytical-Technology-PAT-Tools-to-Early-Active-Pharmaceutical-Ingredient-API-Development/. Accessed 19 Jan 2016
Cervera-Padrell AE, Nielsen JP, Jønch Pedersen M, Müller Christensen K, Mortensen AR, Skovby T, et al. Monitoring and control of a continuous Grignard reaction for the synthesis of an active pharmaceutical ingredient intermediate using inline NIR spectroscopy. Org Process Res Dev. 2012;16(5):901–14.
Roberto M, Dearing T, Branham C, Bleie O, Marquardt B. Rapid determination of optimal conditions in a continuous flow reactor using process analytical technology. Processes. 2013;2(1):24–33.
Sellick CA, Hansen R, Jarvis RM, Maqsood AR, Stephens GM, Dickson AJ, et al. Rapid monitoring of recombinant antibody production by mammalian cell cultures using Fourier transform infrared spectroscopy and chemometrics. Biotechnol Bioeng. 2010;106(3):432–42.
Vieira RM, Embiruçu M, Sayer C, Pinto JC, Lima EL. Control strategies for complex chemical processes. Applications in polymerization processes. Comput Chem Eng. 2003;27(8–9):1307–27.
Schaefer C, Lecomte C, Clicq D, Merschaert A, Norrant E, Fotiadu F. On-line near infrared spectroscopy as a Process Analytical Technology (PAT) tool to control an industrial seeded API crystallization. J Pharm Biomed Anal. 2013;83:194–201.
Knop K, Kleinebudde P. PAT-tools for process control in pharmaceutical film coating applications. Int J Pharm. 2013;457(2):527–36.
Saerens L, Segher N, Vervaet C, Remon JP, De Beer T. Validation of an in-line Raman spectroscopic method for continuous active pharmaceutical ingredient quantification during pharmaceutical hot-melt extrusion. Anal Chim Acta. 2014;806:180–7.
De Beer T, Vercruysse P, Burggraeve A, Quinten T, Ouyang J, Zhang X, et al. In-line and real-time process monitoring of a freeze drying process using Raman and NIR spectroscopy as complementary process analytical technology (PAT) tools. J Pharm Sci. 2009;98(9):3430–46.
De Beer T, Burggraeve A, Fonteyne M, Saerens L, Remon JP, Vervaet C. Near infrared and Raman spectroscopy for the in-process monitoring of pharmaceutical production processes. Int J Pharm. 2011;417(1–2):32–47.
Vibrational spectroscopy in pharmaceutical development. Particle Sciences, Technical Brief; 2011;7.
Rajalahti T, Kvalheim OM. Multivariate data analysis in pharmaceutics: a tutorial review. Int J Pharm. 2011;417(1–2):280–90.
Chen Z, Lovett D, Morris J. Process analytical technologies and real time process control a review of some spectroscopic issues and challenges. J Process Control. 2011;21(10):1467–82.
FDA. Guidance for industry guidance for industry PAT — a framework for innovative pharmaceutical. 2004.
Felizardo P, Folque F, Machado JE, Menezes JC. Process analytical technology: a common approach across different industries. NIR News. 2012;23(7):10.
Menezes JC, Ferreira AP, Rodrigues LO, Brás LP, Alves TP. Chemometrics role within the PAT context: examples from primary pharmaceutical manufacturing. In: Brown S, Tauler R, Walczak A, editors. Comprehensive chemometrics, vol. 4. Oxford: Elsevier; 2009. p. 313–55.
Reich G. Near-infrared spectroscopy and imaging: basic principles and pharmaceutical applications. Adv Drug Deliv Rev. 2005;57:1109–43.
Smith E, Dent G. Modern Raman spectroscopy—a practical approach. New York: Wiley; 2005.
Urbach H. US4007221 United States Patent. 1977;7–10.
Igne B, Hurburgh CR. Local chemometrics for samples and variables: optimizing calibration and standardization processes. J Chemom. 2010. doi:10.1002/cem.1274.
Xiaobo Z, Jiewen Z, Povey MJW, Holmes M, Hanpin M. Variable selection methods in near-infrared spectroscopy. Anal Chim Acta. 2010. doi:10.1016/j.aca.2010.03.048.
Montague GA, Martin EB, O’Malley CJ. Forecasting for fermentation operational decision making. Biotechnol Progr. 2008. doi:10.1021/bp.29.
Sandor M, Rüdinger F, Bienert R, Grimm C, Solle D, Scheper T. Comparative study of non-invasive monitoring via infrared spectroscopy for mammalian cell cultivations. J Biotechnol. 2013. doi:10.1016/j.jbiotec.2013.08.002.
Bro R. Multivariate calibration: what is in chemometrics for the analytical chemist? Anal Chim Acta. 2003; doi:10.1016/S0003-2670(03)00681-0.
Wiss J, Zilian A. Online spectroscopic investigations (FT-IR/Raman) of industrial reactions: synthesis of tributyltin azide and hydrogenation of chloronitrobenzene. Org Process Res Dev. 2003. doi:10.1021/op030009w.
Neal NS, Orefuwa AS, Overton TA, Staples JR, Mohamed AA. Synthesis of diazonium tetrachloroaurate(III) precursors for surface grafting. Inorganics. 2013. doi:10.3390/inorganics1010070.
Li T, Huang B, Wang S, Chen C, Tang L, Lu Q, et al. Covalent grafting of organic molecules onto activated carbon by a single step. Bio Resources. 2013;8(2):2300–9.
Wise BM, Roginski RT. A calibration model maintenance roadmap. IFAC-PapersOnLine; 2015. doi:10.106/j.ifacol.2015.08.191.
Strachan CJ, Rades T, Gordon KC, Rantanen J. Raman spectroscopy for quantitative analysis of pharmaceutical solids. J Pharm Pharmacol. 2007. doi:10.1211/jpp.59.2.0005.
Jensen PS, Bak J. Near-infrared transmission spectroscopy of aqueous solutions: influence of optical pathlength on signal-to-noise ratio. Appl Spectrosc. 2002;56(12):1600–6.
Myerson AS, Krumme M, Nasr M, Thomas H, Braatz RD. Control systems engineering in continuous pharmaceutical manufacturing. J Pharm Sci. 2015. doi:10.1002/jps.24311.
Acknowledgments
The authors would like to acknowledge all members of the Process Optimization Laboratory, Chemical Production Development, Chemical Production Denmark, H. Lundbeck A/S.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Additional information
Published in the topical collection Process Analytics in Science and Industry with guest editor Rudolf W. Kessler.
Rights and permissions
About this article
Cite this article
Gouveia, F.F., Rahbek, J.P., Mortensen, A.R. et al. Using PAT to accelerate the transition to continuous API manufacturing. Anal Bioanal Chem 409, 821–832 (2017). https://doi.org/10.1007/s00216-016-9834-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-016-9834-z