Abstract
Novel psychoactive substances (NPS) are ever changing on the drug market, making it difficult for toxicology laboratory methods to stay current with so many new drugs. Recently, PV8, a synthetic pyrrolidinophenone, was detected in seized products in Japan (2013), The Netherlands (2014), and Germany (2014). There are no controlled PV8 administration studies, and no pharmacodynamic and pharmacokinetic data. The objective was to determine PV8’s metabolic stability in human liver microsome (HLM) incubation and its metabolism following human hepatocyte incubation and high-resolution mass spectrometry (HRMS) with a Thermo Scientific Q-Exactive. Data were acquired with a full-scan data-dependent mass spectrometry method. Scans were thoroughly data mined with different data processing algorithms and analyzed in WebMetaBase. PV8 exhibited a relatively short 28.8 min half-life, with an intrinsic 24.2 μL/min/mg microsomal clearance. This compound is predicted to be an intermediate clearance drug with an estimated human 22.7 mL/min/kg hepatic clearance. Metabolic pathways identified in vitro included: hydroxylation, ketone reduction, carboxylation, N-dealkylation, iminium formation, dehydrogenation, N-oxidation, and carbonylation. The top three in vitro metabolic pathways were di-hydroxylation > ketone reduction > γ-lactam formation. Authentic urine specimen analyses revealed the top three metabolic pathways were aliphatic hydroxylation > ketone reduction + aliphatic hydroxylation > aliphatic carboxylation, although the most prominent peak was parent PV8. These data provide useful urinary metabolite targets (aliphatic hydroxylation, aliphatic hydroxylation + ketone reduction, aliphatic carboxylation, and di-hydroxylation) for forensic and clinical testing, and focus reference standard companies’ synthetic efforts to provide commercially available standards needed for PV8 biological specimen testing.

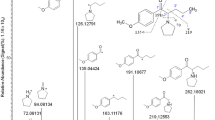
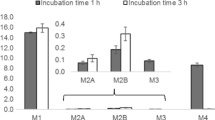
Top four PV8 metabolites identified in vitro. Biotransformations highlighted in blue. Markush structures presented when exact location of biotransformation is unknown




Similar content being viewed by others
References
European Monitoring Center for Drugs and Drug Addiction. New psychoactive substances in Europe—an update from the EU Early Warning System. 2015.
United Nations Office on Drugs and Crime. World Drug Report 2013. Vienna: United Nations; 2013.
Kelly JP. Cathinone derivatives: a review of their chemistry, pharmacology, and toxicology. Drug Test Anal. 2011;3(7/8):439–53.
Gregg RA, Rawls SM. Behavioral pharmacology of designer cathinones: a review of the preclinical literature. Life Sci. 2014;97(1):27–30.
Capriola M. Synthetic cathinone abuse. Clin Pharmacol Adv Appl. 2013;5:109–15.
Gunderson EW, Kirkpatrick MG, Willing LM, Holstege CP. Substituted cathinone products: a new trend in "bath salts" and other designer stimulant drug use. J Addict Med. 2013;7(3):153–62.
Lehner KR, Baumann MH. Psychoactive "bath salts": compounds, mechanisms, and toxicities. Neuropsychopharmacology. 2013;38(1):243–4.
Harrigan T. Schedules of controlled substances: temporary placement of 10 synthetic cathinones into Schedule I. Fed Regist. 2014;79(45):12938–43.
Uchiyama N, Matsuda S, Kawamura M, Shimokawa Y, Kikura-Hanajiri R, Aritake K, et al. Characterization of four new designer drugs, 5-chloro-NNEI, NNEI indazole analog, alpha-PHPP and alpha-POP, with 11 newly distributed designer drugs in illegal products. Forensic Sci Int. 2014;243C:1–13.
Roesner P. Designer Drugs Online News: PV8 (1-Phenyl-2-(1-pyrrolidinyl)-1-heptanone). The compound PV8 (1-Phenyl-2-(1-pyrrolidinyl)-1-heptanone) has been found by the Dutch Customs Laboratory. Altenholz: DigiLab Software GmbH; 2014.
Roesner P. Designer Drugs Online News: PV-8. The compound PV-8 (2-(Pyrrolidin-1-yl)-1phenylheptan-1-one) has been found by the State Office of Criminal. Altenholz: DigiLab Software GmbH.
Martin T. Report about the number of exhibits containing Emerging Drug compounds from January 2013 to April 2014 analyzed by the Drug Chemistry Branch, USACIL. Personal communication.
Elliott S, Evans J. A 3-year review of new psychoactive substances in casework. Forensic Sci Int. 2014;243C:55–60.
Baumann MH, Ayestas Jr MA, Partilla JS, Sink JR, Shulgin AT, Daley PF, et al. The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue. Neuropsychopharmacology. 2012;37(5):1192–203.
Baumann MH, Partilla JS, Lehner KR, Thorndike EB, Hoffman AF, Holy M, et al. Powerful cocaine-like actions of 3,4-methylenedioxypyrovalerone (MDPV), a principal constituent of psychoactive “bath salts” products. Neuropsychopharmacology. 2013;38(4):552–62.
Marusich JA, Antonazzo KR, Wiley JL, Blough BE, Partilla JS, Baumann MH (2014) Pharmacology of novel synthetic stimulants structurally related to the “bath salts” constituent 3,4-methylenedioxypyrovalerone (MDPV). Neuropharmacology. 2014;87:206–13.
Leach AG. Predicting the activity and toxicity of new psychoactive substances: a pharmaceutical industry perspective. Drug Test Anal. 2014;6(7/8):739–45.
Springer D, Peters FT, Fritschi G, Maurer HH. Studies on the metabolism and toxicological detection of the new designer drug 4'-methyl-alpha-pyrrolidinopropiophenone in urine using gas chromatography-mass spectrometry. J Chromatogr B. 2002;773(1):25–33.
Springer D, Staack RF, Paul LD, Kraemer T, Maurer HH. Identification of cytochrome P450 enzymes involved in the metabolism of 4'-methoxy-alpha-pyrrolidinopropiophenone (MOPPP), a designer drug, in human liver microsomes. Xenobiotica. 2003;33(10):989–98.
Springer D, Fritschi G, Maurer HH. Metabolism of the new designer drug alpha-pyrrolidinopropiophenone (PPP) and the toxicological detection of PPP and 4'-methyl-alpha-pyrrolidinopropiophenone (MPPP) studied in rat urine using gas chromatography-mass spectrometry. J Chromatogr B. 2003;796(2):253–66.
Meyer MR, Du P, Schuster F, Maurer H. Studies on the metabolism of the α-pyrrolidinophenone designer drug methylenedioxy-pyrovalerone (MDPV) in rat and human urine and human liver microsomes using GC-MS and LC-high-resolution MS and its detectability in urine by GC-MS. J Mass Spectrom. 2010;45(12):1426–42.
Sauer C, Peters FT, Haas C, Meyer MR, Fritschi G, Maurer HH. New designer drug α-pyrrolidinovalerophenone (PVP): studies on its metabolism and toxicological detection in rat urine using gas chromatographic/mass spectrometric techniques. J Mass Spectrom. 2009;44(6):952–64.
Springer D, Peters FT, Fritschi G, Maurer HH. New designer drug 4'-methyl-alpha-pyrrolidinohexanophenone: studies on its metabolism and toxicological detection in urine using gas chromatography-mass spectrometry. J Chromatogr B. 2003;789(1):79–791.
Zaitsu K, Katagi M, Tsuchihashi H, Ishii A. Recently abused synthetic cathinones, α-pyrrolidinophenone derivatives: a review of their pharmacology, acute toxicity, and metabolism. Forensic Toxicol. 2014;32(1):1–8.
Wohlfarth A, Pang S, Zhu M, Gandhi AS, Scheidweiler KB, Liu HF, et al. First metabolic profile of XLR-11, a novel synthetic cannabinoid, obtained by using human hepatocytes and high-resolution mass spectrometry. Clin Chem. 2013;53(3):423–34.
Gandhi AS, Zhu M, Pang S, Wohlfarth A, Scheidweiler KB, Liu HF (2013) First characterization of AKB-48 metabolism, a novel synthetic cannabinoid, using human hepatocytes and high-resolution mass spectrometry. AAPS J. 2013;15(4):1091–8.
Ellefsen K, Wohlfarth A, Swortwood M, Diao X, Concheiro M, Huestis M. 4-Methoxy-α-PVP: in silico prediction, metabolic stability, and metabolite identification by human hepatocyte incubation and high-resolution mass spectrometry. Forensic Toxicol. 2016;34(1):61–75.
Diao X, Wohlfarth A, Pang S, Scheidweiler KB, Huestis MA. High-resolution mass spectrometry for characterizing the metabolism of synthetic cannabinoid THJ-018 and its 5-hluoro analog THJ-2201 after incubation in human hepatocytes. Clin Chem. 2016;62(1):157–69.
Li AP. Human hepatocytes: isolation, cryopreservation and applications in drug development. Chem Biol Interact. 2007;168(1):16–29.
Swortwood MJ, Carlier J, Ellefsen KN, Wohlfarth A, Diao X, Concheiro-Guisan M, et al. In vitro, in vivo and in silico metabolic profiling of α-pyrrolidinopentiothiophenone, a novel thiophene stimulant. Bioanalysis. 2016;8(1):65–82.
Baranczewski P, Stanczak A, Sundberg K, Svensson R, Wallin A, Jansson J, et al. Introduction to in vitro estimation of metabolic stability and drug interactions of new chemical entities in drug discovery and development. Pharmacol Rep. 2006;58(4):453–72.
McNaney CA, Drexler DM, Hnatyshyn SY, Zvyaga TA, Knipe JO, Belcastro JV, et al. An automated liquid chromatography-mass spectrometry process to determine metabolic stability half-life and intrinsic clearance of drug candidates by substrate depletion. Assay Drug Dev Technol. 2008;6(1):121–9.
Backfisch G, Reder-Hilz B, Hoeckels-Messemer J, Angstenberger J, Sydor J, Laplanche L, et al. High-throughput quantitative and qualitative analysis of microsomal incubations by cocktail analysis with an ultraperformance liquid chromatography-quadrupole time-of-flight mass spectrometer system. Bioanalysis. 2015;7(6):671–83.
Concheiro M, Anizan S, Ellefsen K, Huestis MA. Simultaneous quantification of 28 synthetic cathinones and metabolites in urine by liquid chromatography-high resolution mass spectrometry. Anal Bioanal Chem. 2013;405(29):9437–48.
Shima N, Katagi M, Kamata H, Matsuta S, Sasaki K, Kamata T, et al. Metabolism of the newly encountered designer drug α-pyrrolidinovalerophenone in humans: identification and quantitation of urinary metabolites. Forensic Toxicol. 2014;32(1):59–67.
Tyrkko E, Pelander A, Ketola RA, Ojanpera I. In silico and in vitro metabolism studies support identification of designer drugs in human urine by liquid chromatography/quadrupole-time-of-flight mass spectrometry. Anal Bioanal Chem. 2013;405(21):6697–709.
Negreira N, Erratico C, Kosjek T, van Nuijs AL, Heath E, Neels H, et al. In vitro phase I and phase II metabolism of alpha-pyrrolidinovalerophenone (alpha-PVP), methylenedioxypyrovalerone (MDPV) and methedrone by human liver microsomes and human liver cytosol. Anal Bioanal Chem. 2015;407(19):5803–16.
Meyer MR, Wilhelm J, Peters FT, Maurer HH. Beta-keto amphetamines: studies on the metabolism of the designer drug mephedrone and toxicological detection of mephedrone, butylone, and methylone in urine using gas chromatography-mass spectrometry. Anal Bioanal Chem. 2010;397(3):1225–33.
Lavé T, Dupin S, Schmitt C, Valles B, Ubeaud G, Chou RC, et al. The use of human hepatocytes to select compounds based on their expected hepatic extraction ratios in humans. Pharm Res. 1997;14(2):152–5.
Strano-Rossi S, Cadwallader AB, de la Torre X, Botre F. Toxicological determination and in vitro metabolism of the designer drug methylenedioxypyrovalerone (MDPV) by gas chromatography/mass spectrometry and liquid chromatography/quadrupole time-of-flight mass spectrometry. Rapid Commun Mass Spectrom. 2010;24(18):2706–14.
Namera A, Konuma K, Kawamura M, Saito T, Nakamoto A, Yahata M, et al. Time-course profile of urinary excretion of intravenously administered α-pyrrolidinovalerophenone and α-pyrrolidinobutiophenone in a human. Forensic Toxicol. 2014;32(1):68–74.
Shima N, Kakehashi H, Matsuta S, Kamata H, Nakano S, Sasaki K, et al. Urinary excretion and metabolism of the α-pyrrolidinophenone designer drug 1-phenyl-2-(pyrrolidin-1-yl)octan-1-one (PV9) in humans. Forensic Toxicol. 2015;33:279–94.
Acknowledgments
The authors thank Tim Moeller of Bioreclamation IVT for his assistance with the hepatocyte incubation, as well as Ismael Zamora and his team at Molecular Discovery for the MetaSite and WebMetabase software. This research was funded by the Intramural Research Program of the National Institute on Drug Abuse, National Institutes of Health.
Conflict of interest
The authors have no conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 822 kb)
Rights and permissions
About this article
Cite this article
Swortwood, M.J., Ellefsen, K.N., Wohlfarth, A. et al. First metabolic profile of PV8, a novel synthetic cathinone, in human hepatocytes and urine by high-resolution mass spectrometry. Anal Bioanal Chem 408, 4845–4856 (2016). https://doi.org/10.1007/s00216-016-9599-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-016-9599-4