Abstract
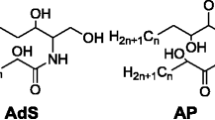
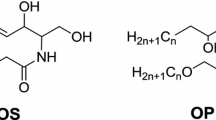
The stratum corneum (SC) is the outermost layer of skin that functions as a barrier and protects against environmental influences and transepidermal water loss. Its unique morphology consists of keratin-enriched corneocytes embedded in a distinctive mixture of lipids containing mainly ceramides, free fatty acids, and cholesterol. Ceramides are sphingolipids consisting of sphingoid bases, which are linked to fatty acids by an amide bond. Typical sphingoid bases in the skin are composed of dihydrosphingosine (dS), sphingosine (S), phytosphingosine (P), and 6-hydroxysphingosine (H), and the fatty acid acyl chains are composed of non-hydroxy fatty acid (N), α-hydroxy fatty acid (A), ω-hydroxy fatty acid (O), and esterified ω-hydroxy fatty acid (E). The 16 ceramide classes include several combinations of sphingoid bases and fatty acid acyl chains. Among them, N-type ceramides are the most abundant in the SC. Mass spectrometry (MS)/MS analysis of N-type ceramides using chip-based direct infusion nanoelectrospray-ion trap mass spectrometry generated the characteristic fragmentation pattern of both acyl and sphingoid units, which could be applied to structural identification of ceramides. Based on the MS/MS fragmentation patterns of N-type ceramides, comprehensive fragmentation schemes were proposed. In addition, mass fragmentation patterns, which are specific to the sphingoid backbone of N-type ceramides, were found in higher m/z regions of tandem mass spectra. These characteristic and general fragmentation patterns were used to identify N-type ceramides in human SC. Based on established MS/MS fragmentation patterns of N-type ceramides, 52 ceramides (including different classes of NS, NdS, NP, and NH) were identified in human SC. The MS/MS fragmentation patterns of N-type ceramides were characterized by interpreting their product ion scan mass spectra. This information may be used to identify N-type ceramides in the SC of human, rat, and mouse skin.










Similar content being viewed by others
References
Masukawa Y, Narita H, Shimizu E, Kondo N, Sugai Y, Oba T, Homma R, Ishikawa J, Takagi Y, Kitahara T, Takema Y, Kita K (2008) Characterization of overall ceramide species in human stratum corneum. J Lipid Res 49(7):1466–1476. doi:10.1194/jlr.M800014-JLR200
t’Kindt R, Jorge L, Dumont E, Couturon P, David F, Sandra P, Sandra K (2012) Profiling and characterizing skin ceramides using reversed-phase liquid chromatography-quadrupole time-of-flight mass spectrometry. Anal Chem 84(1):403–411. doi:10.1021/ac202646v
Weerheim A, Ponec M (2001) Determination of stratum corneum lipid profile by tape stripping in combination with high-performance thin-layer chromatography. Arch Dermatol Res 293(4):191–199
Mizutani Y, Mitsutake S, Tsuji K, Kihara A, Igarashi Y (2009) Ceramide biosynthesis in keratinocyte and its role in skin function. Biochimie 91(6):784–790. doi:10.1016/j.biochi.2009.04.001
Lampe MA, Burlingame AL, Whitney J, Williams ML, Brown BE, Roitman E, Elias PM (1983) Human stratum corneum lipids: characterization and regional variations. J Lipid Res 24(2):120–130
Holleran WM, Takagi Y, Uchida Y (2006) Epidermal sphingolipids: metabolism, function, and roles in skin disorders. FEBS Lett 580(23):5456–5466. doi:10.1016/j.febslet.2006.08.039
Motta S, Monti M, Sesana S, Caputo R, Carelli S, Ghidoni R (1993) Ceramide composition of the psoriatic scale. Biochim Biophys Acta 1182(2):147–151
Jungersted JM, Scheer H, Mempel M, Baurecht H, Cifuentes L, Hogh JK, Hellgren LI, Jemec GB, Agner T, Weidinger S (2010) Stratum corneum lipids, skin barrier function and filaggrin mutations in patients with atopic eczema. Allergy 65(7):911–918. doi:10.1111/j.1398-9995.2010.02326.x
Higuchi H, Nakamura M, Kuwano A, Kasamatsu M, Nagahata H (2005) Quantities and types of ceramides and their relationships to physical properties of the horn covering the claws of clinically normal cows and cows with subclinical laminitis. Can J Vet Res 69(2):155–158
Vietzke JP, Brandt O, Abeck D, Rapp C, Strassner M, Schreiner V, Hintze U (2001) Comparative investigation of human stratum corneum ceramides. Lipids 36(3):299–304
Sahle FF, Lange S, Dobner B, Wohlrab J, Neubert RH (2012) Development and validation of LC/ESI-MS method for the detection and quantification of exogenous ceramide NP in stratum corneum and other layers of the skin. J Pharm Biomed Anal 60:7–13. doi:10.1016/j.jpba.2011.10.032
Raith K, Neubert RHH (2000) Liquid chromatography–electrospray mass spectrometry and tandem mass spectrometry of ceramides. Anal Chim Acta 403:295–303. doi:10.1016/s0003-2670(99)00661-3
Hsu FF, Turk J (2002) Characterization of ceramides by low energy collisional-activated dissociation tandem mass spectrometry with negative-ion electrospray ionization. J Am Soc Mass Spectrom 13(5):558–570. doi:10.1016/S1044-0305(02)00358-6
Hinder A, Schmelzer CE, Rawlings AV, Neubert RH (2011) Investigation of the molecular structure of the human stratum corneum ceramides [NP] and [EOS] by mass spectrometry. Skin Pharmacol Physiol 24(3):127–135. doi:10.1159/000322303
Farwanah H, Pierstorff B, Schmelzer CE, Raith K, Neubert RH, Kolter T, Sandhoff K (2007) Separation and mass spectrometric characterization of covalently bound skin ceramides using LC/APCI-MS and Nano-ESI-MS/MS. J Chromatogr B Anal Technol Biomed Life Sci 852(1–2):562–570. doi:10.1016/j.jchromb.2007.02.030
Zhang J, Yan L, Chen W, Lin L, Song X, Yan X, Hang W, Huang B (2009) Metabonomics research of diabetic nephropathy and type 2 diabetes mellitus based on UPLC-oaTOF-MS system. Anal Chim Acta 650(1):16–22. doi:10.1016/j.aca.2009.02.027
Wang Y, Zhang H (2011) Tracking phospholipid profiling of muscle from Ctennopharyngodon idellus during storage by shotgun lipidomics. J Agric Food Chem 59(21):11635–11642. doi:10.1021/jf2030852
Shen Q, Wang Y, Gong L, Guo R, Dong W, Cheung HY (2012) Shotgun lipidomics strategy for fast analysis of phospholipids in fisheries waste and its potential in species differentiation. J Agric Food Chem 60(37):9384–9393. doi:10.1021/jf303181s
Schuhmann K, Herzog R, Schwudke D, Metelmann-Strupat W, Bornstein SR, Shevchenko A (2011) Bottom-up shotgun lipidomics by higher energy collisional dissociation on LTQ Orbitrap mass spectrometers. Anal Chem 83(14):5480–5487. doi:10.1021/ac102505f
Heiskanen LA, Suoniemi M, Ta HX, Tarasov K, Ekroos K (2013) Long-term performance and stability of molecular shotgun lipidomic analysis of human plasma samples. Anal Chem. doi:10.1021/ac401857a
Han X (2002) Characterization and direct quantitation of ceramide molecular species from lipid extracts of biological samples by electrospray ionization tandem mass spectrometry. Anal Biochem 302(2):199–212. doi:10.1006/abio.2001.5536
Raith K, Neubert RHH (1998) Structural studies on ceramides by electrospray tandem mass spectrometry. Rapid Comm Mass Spectrom 12:935–938. doi:10.1002/(sici)1097-0231(19980731)12:14<935::aid-rcm260>3.0.co;2-u
Bonté F, Saunois A, Pinguet P, Meybeck A (1997) Existence of a lipid gradient in the upper stratum corneum and its possible biological significance. Arch Dermatol Res 289(2):78–82
Jiang X, Cheng H, Yang K, Gross RW, Han X (2007) Alkaline methanolysis of lipid extracts extends shotgun lipidomics analyses to the low-abundance regime of cellular sphingolipids. Anal Biochem 371(2):135–145. doi:10.1016/j.ab.2007.08.019
Gu M, Kerwin JL, Watts JD, Aebersold R (1997) Ceramide profiling of complex lipid mixtures by electrospray ionization mass spectrometry. Anal Biochem 244(2):347–356. doi:10.1006/abio.1996.9915
van Smeden J, Hoppel L, van der Heijden R, Hankemeier T, Vreeken RJ, Bouwstra JA (2011) LC/MS analysis of stratum corneum lipids: ceramide profiling and discovery. J Lipid Res 52(6):1211–1221. doi:10.1194/jlr.M014456
Acknowledgments
This work was supported by the Korea Healthcare Technology R&D Project, Ministry of Health and Welfare (grant A103017), Republic of Korea.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Shin, JH., Shon, J.C., Lee, K. et al. A lipidomic platform establishment for structural identification of skin ceramides with non-hydroxyacyl chains. Anal Bioanal Chem 406, 1917–1932 (2014). https://doi.org/10.1007/s00216-013-7601-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-013-7601-y