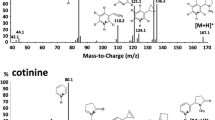
Abstract
The nicotine metabolites, cotinine and trans-3′-hydroxycotinine (3HC) are considered as superior biomarkers for identifying tobacco exposure. More importantly, the ratio of 3HC to cotinine is a good indicator to phenotype individuals for cytochrome P450 2A6 activity and to individualize pharmacotherapy for tobacco addiction. In this paper, a simple, robust and novel method based on surface-enhanced Raman spectroscopy coupled with thin-layer chromatography (TLC) was developed to directly quantify the biomarkers in human urine samples. This is the first time surface-enhanced Raman spectroscopy (SERS) was used to detect cotinine and 3HC in urine samples. The linear dynamic range for the detection of cotinine is from 40 nM to 8 μM while that of 3HC is from 1 μM to 15 μM. The detection limits are 10 nM and 0.2 μM for cotinine and 3HC, respectively. The proposed method was further validated by quantifying the concentration of both cotinine and 3HC in smokers’ urine samples. This TLC-SERS method allows the direct detection of cotinine in the urine samples of both active and passive smokers and the detection of 3HC in smokers.

Scheme of the procedure for detection of cotinine and 3HC





Similar content being viewed by others
References
WHO Library Cataloguing (2008) World Health Organization, Switzerland. www.who.int/evidence/bod. Accessed 29 Oct 2008
Benowitz NL, Jacob P (1994) Metabolism of nicotine to cotinine studied by a dual stable isotope method. Clin Pharmacol Ther 56:483–493
Messina E, Tyndale R, Sellers E (1997) A major role for CYP2A6 in nicotine C-oxidation by human liver microsomes. J Pharmacol Exp Ther 282:1608–1614
Nakajima M, Yamamoto T, Nunoya K, Yokoi T, Nagashima K, Inoue K, Funae Y, Shimada N, Kamataki T, Kuroiwa Y (1996) Characterization of CYP2A6 involved in 3′-hydroxylation of cotinine in human liver microsomes. J Pharmacol Exp Ther 277:1010–1015
Benowitz N, Jacob P III (2001) Trans-3′-hydroxycotinine: disposition kinetics, effects and plasma levels during cigarette smoking. Br J Clin Pharmacol 51:53–59
Gorrod JW, Jacob P (1999) Analytical determination of nicotine and related compounds and their metabolities. Elsevier, Netherlands
Lafay F, Vulliet E, Flament-Waton M-M (2010) Contribution of microextraction in packed sorbent for the analysis of cotinine in human urine by GC–MS. Anal Bioanal Chem 396:937–941
Shakleya DM, Huestis MA (2009) Optimization and validation of a liquid chromatography-tandem mass spectrometry method for the simultaneous quantification of nicotine, cotinine, trans-3′-hydroxycotinine and norcotinine in human oral fluid. Anal Bioanal Chem 395:2349–2357
Hariharan M, VanNoord T, Greden JF (1988) A high-performance liquid-chromatographic method for routine simultaneous determination of nicotine and cotinine in plasma. Clin Chem 34:724–729
Gonzalez JM, Foley MW, Bieber NM, Bourdelle PA, Niedbala RS (2011) Development of an ultrasensitive immunochromatography test to detect nicotine metabolites in oral fluids. Anal Bioanal Chem 400:3655–3664
Kneipp K, Wang Y, Kneipp H, Perelman LT, Itzkan I, Dasari RR, Feld MS (1997) Single molecule detection using surface-enhanced Raman scattering (SERS). Phys Rev Lett 78:1667–1670
Nie S, Emory SR (1997) Probing single molecules and single nanoparticles by surface-enhanced Raman scattering. Science 275:1102–1106
Barber TE, List MS, Haas JW, Wachter EA (1994) Determination of nicotine by surface-enhanced Raman scattering (SERS). Appl Spectrosc 48:1423–1427
Mamián-López MB, Poppi RJ (2013) Standard addition method applied to the urinary quantification of nicotine in the presence of cotinine and anabasine using surface enhanced Raman spectroscopy and multivariate curve resolution. Anal Chim Acta 760:53–59
Lee P, Meisel D (1982) Adsorption and surface-enhanced Raman of dyes on silver and gold sols. J Phys Chem 86:3391–3395
Pal T, Anantha Narayanan V, Stokes D, Vo-Dinh T (1998) Surface-enhanced Raman detection of nicotinamide in vitamin tablets. Anal Chim Acta 368:21–28
Castro JL, Arenas JF, Lopez-Ramirez MR, Soto J, Otero JC (2013) Surface-enhanced Raman scattering of picolinamide, nicotinamide and isonicotinamide: unusual carboxamide deprotonation under adsorption on silver nanoparticles. J Colloid Interface Sci, in press
Stamplecoskie KG, Scaiano JC, Tiwari VS, Anis H (2011) Optimal size of silver nanoparticles for surface-enhanced Raman spectroscopy. J Phys Chem C 115:1403–1409
Stokes RJ, Macaskill A, Lundahl PJ, Smith WE, Faulds K, Graham D (2007) Quantitative enhanced Raman scattering of labeled DNA from gold and silver nanoparticles. Small 3:1593–1601
Kneipp J, Kneipp H, Wittig B, Kneipp K (2010) Novel optical nanosensors for probing and imaging live cells. Nanomed-Nanotechnol 6:214–226
Kahl M, Voges E (2000) Analysis of plasmon resonance and surface-enhanced Raman scattering on periodic silver structures. Phys Rev B 61:14078–14088
Félidj N, Aubard J, Lévi G, Krenn J, Hohenau A, Schider G, Leitner A, Aussenegg F (2003) Optimized surface-enhanced Raman scattering on gold nanoparticle arrays. Appl Phys Lett 82:3095–3097
Le Ru E, Blackie E, Meyer M, Etchegoin P (2007) Surface enhanced Raman scattering enhancement factors: a comprehensive study. J Phys Chem C 111:13794–13803
Lu L, Zhang H, Sun G, Xi S, Wang H, Li X, Wang X, Zhao B (2003) Aggregation-based fabrication and assembly of roughened composite metallic nanoshells: application in surface-enhanced Raman scattering. Langmuir 19:9490–9493
Aroca R, Alvarez-Puebla R, Pieczonka N, Sanchez-Cortez S, Garcia-Ramos J (2005) Surface-enhanced Raman scattering on colloidal nanostructures. Adv Colloid Interface Sci 116:45–61
Dou X, Jung YM, Yamamoto H, Doi S, Ozaki Y (1999) Near-infrared excited surface-enhanced Raman scattering of biological molecules on gold colloid I: effects of pH of the solutions of amino acids and of their polymerization. Appl Spectrosc 53:133–138
István K, Keresztury G, Szép A (2003) Normal Raman and surface enhanced Raman spectroscopic experiments with thin layer chromatography spots of essential amino acids using different laser excitation sources. Spectrochim Acta Part A 59:1709–1723
Song L, Davis W, Abrams SM, Hemiup J, Latif Kazim A, Michael Cummings K, Mahoney MC (2005) Sensitive and rapid method for the determination of urinary cotinine in non-smokers: an application for studies assessing exposures to second hand smoke (SHS). Anal Chim Acta 545:200–208
West O, Hajek P, McRobbie H (2011) Systematic review of the relationship between the 3-hydroxycotinine/cotinine ratio and cigarette dependence. Psychopharmacology 218:313–322
Jarvis MJ, Tunstall-Pedoe H, Feyerabend C, Vesey C, Saloojee Y (1987) Comparison of tests used to distinguish smokers from nonsmokers. Am J Public Health 77:1435–1438
Acknowledgement
This research was supported by James & Esther King Biomedical Research Program, Florida Department of Health. The author also thanks Dr. David Drobes from Moffitt Cancer Center for the urine samples.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 443 kb)
Rights and permissions
About this article
Cite this article
Huang, R., Han, S. & Li, X. Detection of tobacco-related biomarkers in urine samples by surface-enhanced Raman spectroscopy coupled with thin-layer chromatography. Anal Bioanal Chem 405, 6815–6822 (2013). https://doi.org/10.1007/s00216-013-7107-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-013-7107-7