Abstract
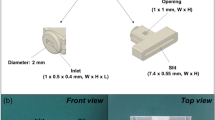
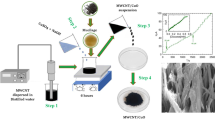
A flexible electrochemical micro(bio)sensor has been designed for determination of several biological compounds, specifically, ascorbate, dopamine, and glucose, in human lachrymal liquid (tears). The microsensor for simultaneous determination of ascorbate and dopamine concentrations was based on a gold microwire modified with the tetrathiafulvalen–7,7,8,8-tetracyanoquinodimethane complex as a catalyst. To monitor glucose concentration in tears, glucose dehydrogenase was immobilized on a gold microwire modified with carbon nanotubes and an osmium redox polymer. A capillary microcell was constructed for sampling tears. The cell had a working volume of 60–100 nL with a sampling deviation of 6.7 %. To check if the microcell was properly filled with buffer or tear sample, a control electrode was introduced into the construction. The electrode was used to measure the electrical resistance of a fully filled nanovolume cell. The mechanical flexibility is one of the most important features of the prototype and allowed direct collection of tears with minimized risk of damage to the eye.

Tear sampling and electrochemical analysis





Similar content being viewed by others
References
PricewaterhouseCoopers’ Health Research Institute (2010) HealthCast: The customization of diagnosis, care and cure. PricewaterhouseCoopers LLP, Delaware, pp 1–56
Cardosi MF, Turner APF (1990) In: Alberti KGMM, Krall LP (eds) The diabetes annual, vol 5. Elsevier, Amsterdam, pp 254–272
Turner APF (1993) Modified enzyme electrodes. In: Guilbault GG, Mascini M (eds) Uses of immobilized biological compounds. NATO ASI series, series E: applied sciences, vol 252. Kluwer, Dordrecht, pp 263–269
Turner APF (2000) Biosensors–sense and sensitivity. Science 290(5495):1315–1317
Lee TM-H (2008) Over-the-counter biosensors: past, present, and future. Sensors 8(9):5535–5559
Davies RJ, Eapen SS, Carlisle SJ (2007) Lateral-flow immunochromatographic assays. Handb Biosens Biochips 2:1151–1165
Weetall HH (2007) Electrochemical polymerization for preparation of electrochemical sensors. Handb Biosens Biochips 1:251–257
Yamaguchi M (2010) Salivary sensors in point-of-care testing. Sens Mater 22(4):143–153
Yao HF, Shum AJ, Cowan M, Lahdesmaki I, Parviz BA (2011) A contact lens with embedded sensor for monitoring tear glucose level. Biosens Bioelectron 26(7):3290–3296
Mitsubayashi K, Dicks JM, Yokoyama K, Takeuchi T, Tamiya E, Karube I (1995) A flexible biosensor for glucose. Electroanalysis 7(1):83–87
Choy CKM, Benzie IFF, Cho P (2003) Antioxidants in tears and plasma: Inter-relationships and effect of vitamin C supplementation. Curr Eye Res 27(1):55–60
Van Haeringen NJ, Glasius E (1977) Collection method-dependent concentrations of some metabolites in human tear fluid, with special reference to glucose in hyperglycemic conditions. Albrecht Von Graefes Arch Klin Exp Ophthalmol 202(1):1–7
Shoham A, Hadziahmetovic M, Dunaief JL, Mydlarski MB, Schipper HM (2008) Oxidative stress in diseases of the human cornea. Free Radic Biol Med 45(8):1047–1055
Crouch RK, Goletz P, Snyder A, Coles WH (1991) Antioxidant enzymes in human tears. J Ocul Pharmacol 7(3):253–258
Gogia R, Richer SP, Rose RC (1998) Tear fluid content of electrochemically active components including water soluble antioxidants. Curr Eye Res 17(3):257–263
Reiss GR, Werness PG, Zollman PE, Brubaker RF (1986) Ascorbic-acid levels in the aqueous-humor of nocturnal and diurnal mammals. AMA Arch Ophthalmol 104(5):753–755
Luxneuwirth O, Millar TJ (1990) Lipid soluble antioxidants preserve rabbit corneal cell-function. Curr Eye Res 9(2):103–109
Vanhaeringen NJ (1981) Clinical biochemistry of tears. Surv Ophthalmol 26(2):84–96
Martin X, Brennan M (1993) Dopamine and its metabolites in human tears. Eur J Ophthalmol 3(2):83–88
Gasset AR, Braverma L, Fleming MC, Arky RA, Alter BR (1968) Tear glucose detection of hyperglycemia. Am J Ophthalmol 65(3):414–420
Chen R, Jin Z, Colon LA (1996) Analysis of tear fluid by CE/LIF: a noninvasive approach for glucose monitoring. J Capillary Electrophor 3(5):243–248
Taormina CR, Baca JT, Asher SA, Grabowski JJ, Finegold DN (2007) Analysis of tear glucose concentration with electrospray ionization mass spectrometry. J Am Soc Mass Spectrom 18(2):332–336
Baca JT, Taormina CR, Feingold E, Finegold DN, Grabowski JJ, Asher SA (2007) Mass spectral determination of fasting tear glucose concentrations in nondiabetic volunteers. Clin Chem 53(7):1370–1372
Alexeev VL, Das S, Finegold DN, Asher SA (2004) Photonic crystal glucose-sensing material for noninvasive monitoring of glucose in tear fluid. Clin Chem 50(12):2353–2360
Badugu R, Lakowicz JR, Geddes CD (2005) Fluorescence sensors for monosaccharides based on the 6-methylquinolinium nucleus and boronic acid moiety: potential application to ophthalmic diagnostics. Talanta 65(3):762–768
Chu MX, Miyajima K, Takahashi D, Arakawa T, Sano K, Sawada S, Kudo H, Iwasaki Y, Akiyoshi K, Mochizuki M, Mitsubayashi K (2011) Soft contact lens biosensor for in situ monitoring of tear glucose as non-invasive blood sugar assessment. Talanta 83(3):960–965
El-Said WA, Lee JH, Oh BK, Choi JW (2010) 3-D nanoporous gold thin film for the simultaneous electrochemical determination of dopamine and ascorbic acid. Electrochem Commun 12(12):1756–1759
Hayashi K, Iwasaki Y, Kurita R, Sunagawa K, Niwa O (2003) On-line microfluidic sensor integrated with a micro array electrode and enzyme-modified pre-reactor for the real-time monitoring of blood catecholamine. Electrochem Commun 5(12):1037–1042
Kalimuthu P, John SA (2009) Electropolymerized film of functionalized thiadiazole on glassy carbon electrode for the simultaneous determination of ascorbic acid, dopamine and uric acid. Bioelectrochemistry 77(1):13–18
Liu AH, Honma I, Zhou HS (2005) Electrochemical biosensor based on protein-polysaccharide hybrid for selective detection of nanomolar dopamine metabolite of 3,4-dihydroxyphenylacetic acid (DOPAC). Electrochem Commun 7(2):233–236
Liu Y, Huang JS, Hou HQ, You TY (2008) Simultaneous determination of dopamine, ascorbic acid and uric acid with electrospun carbon nanofibers modified electrode. Electrochem Commun 10(10):1431–1434
Manjunatha R, Suresh GS, Melo JS, D'Souza SF, Venkatesha TV (2010) Simultaneous determination of ascorbic acid, dopamine and uric acid using polystyrene sulfonate wrapped multiwalled carbon nanotubes bound to graphite electrode through layer-by-layer technique. Sensors Actuators B Chem 145(2):643–650
Raj CR, Okajima T, Ohsaka T (2003) Gold nanoparticle arrays for the voltammetric sensing of dopamine. J Electroanal Chem 543(2):127–133
Pauliukaite R, Malinauskas A, Zhylyak G, Spichiger-Keller UE (2007) Conductive organic complex salt TTF-TCNQ as a mediator for biosensors. An overview. Electroanalysis 19(24):2491–2498
Freund MS, Brajtertoth A, Ward MD (1990) Electrochemical and quartz crystal microbalance evidence for mediation and direct electrochemical reactions of small molecules at tetrathiafulvalene-tetracyanoquinodimethane (TTF-TCNQ) electrodes. J Electroanal Chem 289(1–2):127–141
Khan GF (1996) Construction of SEC/CTC electrodes for direct electron transferring biosensors. Sensors Actuators B Chem 36(1–3):484–490
McKenna K, Brajtertoth A (1987) Tetrathiafulvalene tetracyanoquinodimethane xanthine-oxidase amperometric electrode for the determination of biological purines. Anal Chem 59(7):954–958
Antiochia R, Gorton L (2007) Development of a carbon nanotube paste electrode osmium polymer-mediated biosensor for determination of glucose in alcoholic beverages. Biosens Bioelectron 22(11):2611–2617
Yan QY, Peng B, Su G, Cohan BE, Major TC, Meyerhoff ME (2011) Measurement of tear glucose levels with amperometric glucose biosensor/capillary tube configuration. Anal Chem 83(21):8341–8346
Pundir CS, Chauhan N, Jyoti (2011) Construction of an amperometric ascorbate biosensor using epoxy resin membrane bound Lagenaria siceraria fruit ascorbate oxidase. Artif Cells Blood Substit 39(3):177–184
Paterson CA, Orourke MC (1987) Vitamin-C levels in human tears. AMA Arch Ophthalmol 105(3):376–377
Acknowledgments
The authors thank Lo Gorton for the kind gift of the osmium redox polymer. The work was supported financially by the Faculty of Health and Society, Malmö University, the Swedish Research Council, and the Gustaf Th. Ohlsson Foundation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in the topical collection Bioelectroanalysis with guest editors Nicolas Plumeré, Magdalena Gebala, and Wolfgang Schuhmann.
Rights and permissions
About this article
Cite this article
Andoralov, V., Shleev, S., Arnebrant, T. et al. Flexible micro(bio)sensors for quantitative analysis of bioanalytes in a nanovolume of human lachrymal liquid. Anal Bioanal Chem 405, 3871–3879 (2013). https://doi.org/10.1007/s00216-013-6756-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-013-6756-x