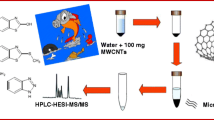
Abstract
Extraction techniques are surface-dependent processes since their kinetic directly depends on the contact area between the sample and the extractant phase. The dispersion of the extractant (liquid or solid) increases this area improving the extraction efficiency. In this article, the dispersion of a nanostructured sorbent at the very low milligram level is achieved by effervescence thanks to the in situ generation of carbon dioxide. For this purpose, a special tablet containing the effervescence precursors (sodium carbonate as carbon dioxide source and sodium dihydrogen phosphate as proton donor) and the sorbent [multiwalled carbon nanotubes (MWCNTs)] is prepared. All the microextraction steps take place in a glass beaker containing 100 mL of the sample. After the extraction, the MWCNTs, enriched with the extracted analytes, are recovered by vacuum filtration. Methanol was selected to elute the retained analytes. The extraction mode is optimized and characterized using the determination of nine herbicides in water samples as model analytical problem. The absolute recoveries of the analytes were in the range 48–76 %, while relative recoveries were close to 100 % in all cases. These values permit the determination of these analytes at the low microgram per liter range with good precision (relative standard deviations lower than 9.3 %) using ultra performance liquid chromatography (UPLC) combined with ultraviolet detection (UV).




Similar content being viewed by others
References
Pyrzynska K (2008) Sep Purif Rev 37:372–389
Valcárcel M, Cárdenas S, Simonet BM (2007) Anal Chem 79:4788–4797
Raveló-Perez LM, Herrera-Herrera AV, Hernández-Borges J, Rodríguez-Delgado MA (2010) J Chromatogr A 1217:2618–2641
Ballesteros E, Gallego M, Valcárcel M (2000) J Chromatogr A 869:101–110
Serrano A, Gallego M (2006) J Sep Sci 29:33–40
Chen WH, Lee SC, Sabu S, Fang HC, Chung SC, Han CC, Chang HC (2006) Anal Chem 78:4228–4234
Wang Y, Gao S, Zang X, Li J, Ma J (2012) Anal Chim Acta 716:112–118
Jiménez-Soto JM, Cárdenas S, Valcárcel M (2009) J Chromatogr A 1216:5626–5633
Jiménez-Soto JM, Cárdenas S, Valcárcel M (2010) J Chromatogr A 1217:3341–3347
Jiménez-Soto JM, Moliner-Martinez Y, Cárdenas S, Valcárcel M (2010) Electrophoresis 31:1681–1688
Jiménez-Soto JM, Cárdenas S, Valcárcel M (2012) Anal Chim Acta 714:76–8
Ijima S (1991) Nature 354:56–58
Cho HH, Smith BA, Wnuk JD, Fairbrother DH, Ball WP (2008) Environ Sci Technol 42:2899–2905
Cai Y, Jiang G, Liu F, Zhou Q (2003) Anal Chem 75:2517–2521
Cruz-Vera M, Lucena R, Cárdenas S, Valcárcel M (2009) Anal Bioanal Chem 391:1139–1145
Carrillo-Carrión C, Lucena R, Cárdenas S, Valcárcel M (2007) Analyst 132:551–559
Menna E, Della Negra F, Prato M, Tagmatarchis N, Ciogli A, Gasparrini F, Misita D, Villani C (2006) Carbon 44:1609–1613
Suarez B, Moliner-Martínez Y, Cárdenas S, Simonet B, Valcárcel M (2008) Environ Sci Technol 42:6100–6104
Anastassiades M, Lehotay SJ, Stajnbaher D, Schenck FJ (2003) J AOAC Int 86:412–431
Alcudia-León MC, Lucena R, Cárdenas S, Valcárcel M (2009) Anal Chem 81:1184–1190
Wang Y, Iqbal Z, Malhotra SV (2005) Chem Phys Lett 402:96–101
Jiang L, Gao L, Sun J (2003) J Colloid Interface Sci 260:89–94
O'Connell MJ, Boul P, Ericson LM, Huffman C, Wang Y, Haroz E (2001) Chem Phys Lett 342:265–271
Bandyopadhyaya R, Nativ-Roth E, Regev O, Yerushalmi-Rozen R (2002) Nano Lett 2:25–28
O'Connell MJ, Bachilo SM, Huffman CB, Moore VC, Strano MS, Haroz EH, Rialon KL, Boul PJ, Noon WH, Kittrell C, Ma J, Hauge H, Weisman RB, Smalley RE (2002) Science 297:593–596
Lu KL, Lago RM, Chen YK, Green MLH, Harris PJF, Tsang SC (1996) Carbon 34:814–816
Pyrzynska K (2011) Chemosphere 83:1407–1413
Jiménez-Soto JM, Cárdenas S, Valcárcel M (2012) J Chromatogr A 1245:17–23
Gebel T, Kevekordes S, Pav K, Edenharder R, Dunkelberg H (1997) Arch Toxicol 71:193–197
Kniewald J, Jakominic M, Tomljenovic A, Simic B, Romac P, Vranesic P, Kniewald Z (2000) J Appl Toxicol 20:61–68
Jiang H, Adams C, Graziano N, Roberson A, McGuire M, Khiari D (2006) Environ Sci Technol 40:3609–3616
LeBaron HM, McFarland JE, Burnside OC (2008) The triazine herbicides. Elsevier, San Diego
Ma WT, Fu KK, Cai Z, Jiang GB (2003) Chemosphere 52:1627–1632
Wu Q, Feng C, Zhao G, Wang C, Wang Z (2012) J Sep Sci 35:193–199
Huff TB, Foster GD (2011) J Environ Sci Health B 46:723–734
Cabrías-Martinez R, Rodriguez-Gonzalo E, Miranda-Cruz E, Dominguez-Alvarez J, Hernandez-Mendez J (2006) J Chromatogr A 1122:194–201
Zhou Q, Xiao J, Wang W, Liu G, Shi Q, Wang J (2006) Talanta 68:1309–1315
Lasarte-Aragones G, Lucena R, Cardenas S, Valcarcel M (2011) J Chromatogr A 1218:9128–9134
US Environmental Protection Agency, Office of Solid Waste (1996) EPA Method 8330, SW-846. Available from NTIS
Smith GA, Pepich BV, Munch DJ (2008) J Chromatogr A 1202:138–144
Shang-Da H, Hsin-I H, Yu-Hsiang S (2004) Talanta 64:887–893
Katsumata H, Kojima H, Kaneco S, Suzuki T, Ohta K (2010) Microchem J 96:348–351
Acknowledgments
Financial support from the Spanish Ministry of Science and Innovation (grant CTQ2011-23790) is gratefully acknowledged. G. Lasarte-Aragonés would like to express his gratitude for the predoctoral grant (ref. AP2009-2850) from the Spanish Ministry of Education.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 156 KB)
Rights and permissions
About this article
Cite this article
Lasarte-Aragonés, G., Lucena, R., Cárdenas, S. et al. Effervescence-assisted carbon nanotubes dispersion for the micro-solid-phase extraction of triazine herbicides from environmental waters. Anal Bioanal Chem 405, 3269–3277 (2013). https://doi.org/10.1007/s00216-013-6718-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-013-6718-3