Abstract
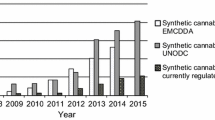
A rapid and simple gas chromatography–mass spectrometry (GC-MS) method was developed and validated to identify and quantify synthetic cannabinoids in the materials seized during drug trafficking. Accuracy and reproducibility of the method were improved by using deuterated JWH-018 and JWH-073 as internal standards. Validation results of the GC-MS method showed that it was suitable for simultaneous qualitative and quantitative analyses of synthetic cannabinoids, and we analyzed synthetic cannabinoids in seized materials using the validated GC-MS method. As a result of the analysis, ten species of synthetic cannabinoids were identified in dried leaves (n = 40), bulk powders (n = 6), and tablets (n = 14) seized in Korea during 2009–2012, as a single ingredient or as a mixture with other active co-ingredients. JWH-018 and JWH-073 were the most frequently identified compounds in the seized materials. Synthetic cannabinoids in the dried leaves showed broad concentration ranges, which may cause unexpected toxicity to abusers. The bulk powders were considered as raw materials used to prepare legal highs, and they contained single ingredient of JWH-073, JWH-019, or JWH-250 with the purity over 70 %. In contrast, JWH-018 and JWH-073 contents in the tablets were 7.1–13.8 and 3.0–10.2 mg/g, respectively. Relatively low contents in the tablets suggest that the synthetic cannabinoids may have been added to the tablets as supplements to other active co-ingredients.


Similar content being viewed by others
References
UNODC (2011) World drug report 2011. United Nations Office of Drugs and Crime (UNODC), Vienna
Aung MM, Griffin G, Huffman JW, Wu MJ, Keel C, Yang B, Showalter VM, Abood ME (2000) Influence of the N-1 alkyl chain length of cannabimimetic indoles upon CB1 and CB2 receptor binding. Drug Alcohol Depend 60:133–140
Huffman JW, Szklennik PV, Almond A, Bushell K, Selley DE, He H, Cassidy MP, Wiley JL, Martin BR (2005) 1-Pentyl-3-phenylacetylindoles, a new class of cannabimimetic indoles. Bioorg Med Chem Lett 15:4110–4113
Auwärter V, Dresen S, Weinmann W, Müller M, Pütz M, Fereirós N (2009) “Spice” and other herbal blends: harmless incense or cannabinoid designer drugs? J Mass Spectrom 44:832–837
Nakajima J, Takahashi M, Seto T, Kanai C, Suzuki J, Yoshida M, Hamano T (2011) Identification and quantitation of two benzylindoles AM-694 and (4-methoxyphenyl)(1-pentyl-1H-indol-3-yl)methanone, and three cannabimimetic naphthoylindoles JWH-210, JWH-122, and JWH-019 as adulterants in illegal products obtained via the internet. Forensic Toxicol 29:95–110
Nakajima J, Takahashi M, Seto T, Suzuki J (2011) Identification and quantitation of cannabimimetic compound JWH-250 as an adulterant in products obtained via the internet. Forensic Toxicol 29:51–55
Uchiyama N, Kawamura M, Kikura-Hanajiri R, Goda Y (2011) Identification and quantitation of two canabimimetic phenylacetylindoles JWH-251 and JWH-250, and four cannabimimetic naphthoylindoles JWH-081, JWH-015, JWH-200, and JWH-073 as designer drugs in illegal products. Forensic Toxicol 29:25–37
Nakajima J, Takahashi M, Nonaka R, Seto T, Suzuki J, Yoshida M, Kanai C, Hamano T (2011) Identification and quantitation of a benzoylindole(2-methoxyphenyl)(1-pentyl-1H-indole-3-yl)-(naphthalene-1-yl)methanone (AM-2201) found in illegal products obtained via the internet and their cannabimimetic effects evaluated by in vitro [35S]GTPγS binding assays. Forensic Toxicol 29:132–141
Ernst L, Schiebel HM, Theuring C, Lindigkeit R, Beuerle T (2011) Identification and characterization of JWH-122 used as new ingredient in “Spice-like” herbal incenses. Forensic Sci Int 208:e31–e35
Moosmann B, Kneisel S, Girreser U, Brecht V, Westphal F, Auwärter V (2012) Separation and structural characterization of the synthetic cannabinoids JWH-412 and 1-[(5-fluoropentyl)-1H-indol-3yl]-(4-methylnaphthalen-1-yl)methanone using GC-MS, NMR analysis and a flash chromatography system. Forensic Sci Int 220:e17–e22
Jankovics P, Váradi A, Tölgyesi L, Lohner S, Németh-Paloás J, Balla J (2012) Detection and identification of the new potential synthetic cannabinoids 1-pentyl-3-(2-indobenzoyl)indole and 1-pentyl-3-(1-adamantoyl)indole in seized bulk powders in Hungary. Forensic Sci Int 214:27–32
Hillebrand J, Olszewski D, Sedefov R (2010) Legal highs on the internet. Subst Use Misuse 45:330–340
Ashton JC (2012) Synthetic cannabinoids as drugs of abuse. Curr Drug Abuse Rev 5:158–168
EMCDDA (2009) Thematic papers: understanding the “Spice” phenomenon. European Monitoring Centre for Drugs and Drug Addiction (EMCDDA), Lisbon
Zimmermann US, Winkelmann PR, Pilhatsch M, Nees JA, Spanagel R, Schulz K (2009) Withdrawal phenomena and dependence syndrome after the consumption of “spice gold”. Dtsch Arztebl Int 106:464–467
Schneir AB, Baumbacher T (2012) Convulsions associated with the use of a synthetic cannabinoid product. J Med Toxicol 8:62–64
Lapoint J, James LP, Moran CL, Nelson LS, Hoffman RS, Moran JH (2011) Severe toxicity following synthetic cannabinoid ingestion. Clin Toxicol (Phila) 49:760–764
Wissenbach DK, Meyer MR, Remane D, Philipp AA, Weber AA, Maurer HH (2011) Drugs of abuse screening in urine as part of a metabolite-based LC-MS screening concept. Anal Bioanal Chem 400:3481–3489
Lindigkeit R, Boehme A, Eiserloh I, Luebbecke M, Wiggermann M (2009) Spice: a never ending story? Forensic Sci Int 191:58–63
Zuba D, Byrska B, Maciow M (2011) Comparison of “herbal highs” composition. Anal Bioanal Chem 400:119–126
Wiskerke J, Stoop N, Schetters D, Schoffelmeer A, Pattij T (2011) Cannabinoid CB1 receptor activation mediates the opposing effects of amphetamine on impulsive action and impulsive choice. PLoS One. doi:10.1371/journal.pone.0025856
Fernández-Ruiz J, Hernández M, Ramos JA (2010) Cannabinoid–dopamine interaction in the pathophysiology and treatment of CNS disorders. CNS Neurosci Ther 16:e72–e91
Acknowledgments
This study was supported by funding from the National R&D program of the Ministry of Education, Science and Technology (2012-0009836) and the National Forensic Service of Korea.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in the special paper collection Forensic Toxicology with guest editors Kazuhito Watanabe and Satoshi Chinaka.
Rights and permissions
About this article
Cite this article
Choi, H., Heo, S., Choe, S. et al. Simultaneous analysis of synthetic cannabinoids in the materials seized during drug trafficking using GC-MS. Anal Bioanal Chem 405, 3937–3944 (2013). https://doi.org/10.1007/s00216-012-6560-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-012-6560-z