Abstract
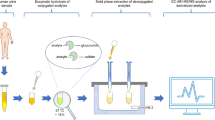
Endocrine disrupting compounds (EDCs) are suspected to be responsible for many disorders of the human reproductive system. To establish a causality relationship between exposure to endocrine disruptors and disease, experiments on animals must be performed with improved or new analytical tools. Therefore, a simple, rapid, and effective multi-residue method was developed for the determination of four steroid hormones (i.e., testosterone, androstenedione, estrone, and estradiol), glucuronide and sulfate conjugates of estrone and estradiol and four endocrine disruptors in rat testis (i.e., bisphenol A, atrazine, and active metabolites of methoxychlor and vinclozolin). The sample preparation procedure was based on the Quick, Easy, Cheap, Effective, Rugged, and Safe (QuEChERS) approach. An analytical method was then developed to quantify these compounds at ultra-trace levels by liquid chromatography coupled to tandem mass spectrometry. The QuEChERS extraction was optimized with regard to the acetonitrile/water ratio used in the extraction step, the choice of the cleanup method and the acetonitrile/hexane ratio used in the cleanup step. The optimized extraction method exhibited recoveries between 89% and 108% for all tested compounds except the conjugates (31% to 58%). The detection limits of all compounds were below 20 ng g−1 of wet weight of testis. The method was subsequently applied to determine the levels of hormones and EDCs in seven rat testis samples.






Similar content being viewed by others
References
Clementi M, Tiboni GM, Causin R, La Rocca C, Maranghi F, Raffagnato F, Tenconi R (2008) Pesticides and fertility: an epidemiological study in Northeast Italy and review of the literature. Reprod Toxicol 26(1):13–18
Chang H-S, Choo K-H, Lee B, Choi S-J (2009) The methods of identification, analysis, and removal of endocrine disrupting compounds (EDCs) in water. J Hazard Mater 172(1):1–12
Ottinger MA, Abdelnabi M, Quinn M, Golden N, Wu J, Thompson N (2002) Reproductive consequences of EDCs in birds: what do laboratory effects mean in field species? Neurotoxicol Teratol 24(1):17–28
Sonnenschein C, Soto AM (1998) An updated review of environmental estrogen and androgen mimics and antagonists. J Steroid Biochem Mol Biol 65(1–6):143–150
Jobling S, Tyler CR (2006) Introduction: the ecological relevance of chemically induced endocrine disruption in wildlife. Environ Health Perspect 114 (S-1)
Sharpe RM, Irvine DS (2004) How strong is the evidence of a link between environmental chemicals and adverse effects on human reproductive health? Br Med J 328(7437):447–451
Kim HS, Lee BM, Jerome ON (2011) Endocrine disrupting chemicals and human cancer. In: Encyclopedia of Environmental Health. Elsevier, Burlington, pp 296–305
Crain DA, Janssen SJ, Edwards TM, Heindel J, Ho S-m, Hunt P, Iguchi T, Juul A, McLachlan JA, Schwartz J, Skakkebaek N, Soto AM, Swan S, Walker C, Woodruff TK, Woodruff TJ, Giudice LC, Guillette LJ Jr (2008) Female reproductive disorders: the roles of endocrine-disrupting compounds and developmental timing. Fertil Steril 90(4):911–940
Petro EML, Covaci A, Leroy JLMR, Dirtu AC, De Coen W, Bols PEJ (2010) Occurrence of endocrine disrupting compounds in tissues and body fluids of Belgian dairy cows and its implications for the use of the cow as a model to study endocrine disruption. Sci Total Environ 408(22):5423–5428
Evans TJ, Ramesh CG, Dvm, Mvsc, Phd, Dabt, Fact (2007) Reproductive toxicity and endocrine disruption. In: Veterinary Toxicology. Academic Press, Oxford, pp 206–244
Milnes MR, Bermudez DS, Bryan TA, Edwards TM, Gunderson MP, Larkin ILV, Moore BC, Guillette JLJ (2006) Contaminant-induced feminization and demasculinization of nonmammalian vertebrate males in aquatic environments. Environ Res 100(1):3–17
Coppock RW (2011) Endocrine disruption in wildlife species. In: Reproductive and Developmental Toxicology. Academic Press, San Diego, pp 1117–1126
Tyler CR, Jobling S, Sumpter JP (1998) Endocrine disruption in wildlife: a critical review of the evidence. Crit Rev Toxicol 28(4):319–361
Rodríguez EM, Medesani DA, Fingerman M (2007) Endocrine disruption in crustaceans due to pollutants: a review. Comp Biochem Physiol-Part A Mol Integr Physiol 146(4):661–671
Ottinger MA, Dean K, Michael DB, Janice M (2010) Vertebrate endocrine disruption. In: Encyclopedia in animal behavior. Academic Press, Oxford, pp 475–484
Labadie P, Budzinski H (2006) Alteration of steroid hormone balance in juvenile turbot exposed to nonylphenol, bisphenol A, tetrabromodiphenyl ether 47, diallylphthalate, oil, and oil spiked with alkylphenols. Arch Environ Contam Toxicol 50(4):552–561
Labadie P, Budzinski H (2006) Alteration of steroid hormone profile in juvenile turbot (Psetta maxima) as a consequence of short-term exposure to 17[alpha]-ethynylestradiol. Chemosphere 64(8):1274–1286
Flores-Valverde AM, Hill EM (2008) Methodology for profiling the steroid metabolome in animal tissues using ultraperformance liquid chromatography-electrospray-time-of-flight mass spectrometry. Anal Chem 80(22):8771–8779
Saudan C, Entenza JM, Baume N, Mangin P, Saugy M (2006) Short-term stability of testosterone and epitestosterone conjugates in urine samples: quantification by liquid chromatography-linear ion trap mass spectrometry. J Chromatogr B 844(1):168–174
Mazzarino M, Rossi F, Giacomelli L, Botrè F (2006) Effect of the systemic versus inhalatory administration of synthetic glucocorticoids on the urinary steroid profile as studied by gas chromatography-mass spectrometry. Anal Chim Acta 559(1):30–36
Strahm E, Kohler I, Rudaz S, Martel S, Carrupt P-A, Veuthey J-L, Saugy M, Saudan C (2008) Isolation and quantification by high-performance liquid chromatography-ion-trap mass spectrometry of androgen sulfoconjugates in human urine. J Chromatogr A 1196–1197:153–160
Marcos V, Perogordo E, Espinosa P, De Pozuelo MM, Hooghuis H (2004) Multiresidue analysis of anabolic compounds in bovine hair by gas chromatography-tandem mass spectrometry. Anal Chim Acta 507(2):219–227
Harwood DT, Handelsman DJ (2009) Development and validation of a sensitive liquid chromatography-tandem mass spectrometry assay to simultaneously measure androgens and estrogens in serum without derivatization. Clin Chim Acta 409(1–2):78–84
Regal P, Vázquez BI, Franco CM, Cepeda A, Fente C (2009) Quantitative LC-MS/MS method for the sensitive and simultaneous determination of natural hormones in bovine serum. J Chromatogr B 877(24):2457–2464
Vulliet E, Wiest L, Baudot R, Grenier-Loustalot M-F (2008) Multi-residue analysis of steroids at sub-ng/L levels in surface and ground-waters using liquid chromatography coupled to tandem mass spectrometry. J Chromatogr A 1210(1):84–91
Tso J, Aga DS (2010) A systematic investigation to optimize simultaneous extraction and liquid chromatography tandem mass spectrometry analysis of estrogens and their conjugated metabolites in milk. J Chromatogr 1217(29):4784–4795
Jantti SE, Tammimaki A, Raattamaa H, Piepponen P, Kostiainen R, Ketola RA (2010) Determination of steroids and their intact glucuronide conjugates in mouse brain by capillary liquid chromatography-tandem mass spectrometry. Anal Chem 82(8):3168–3175
Costain RM, Fesser ACE, McKenzie D, Mizuno M, MacNeil JD (2008) Identification of hormone esters in injection site in muscle tissues by LC/MS/MS. Food Addit Contam 25(12):1520–1529
Stubbings G, Bigwood T (2009) The development and validation of a multiclass liquid chromatography tandem mass spectrometry (LC-MS/MS) procedure for the determination of veterinary drug residues in animal tissue using a QuEChERS (Quick, Easy, Cheap, Effective, Rugged and Safe) approach. Anal Chim Acta 637(1–2):68–78
Anastassiades M, Lehotay S, Stajnbaher D, Schenck F (2003) Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J AOAC Int 86(2):412–431
Martínez Vidal J, Frenich A, Aguilera-Luiz M, Romero-González R (2010) Development of fast screening methods for the analysis of veterinary drug residues in milk by liquid chromatography-triple quadrupole mass spectrometry. Anal Bioanal Chem 397(7):2777–2790
Furlani RPZ, Marcilio KM, Leme FM, Tfouni SAV (2010) Analysis of pesticide residues in sugarcane juice using QuEChERS sample preparation and gas chromatography with electron capture detection. Food Chem 126(3):1283–1287
Pinto CG, Laespada MEF, Martín SH, Ferreira AMC, Pavón JLP, Cordero BM (2009) Simplified QuEChERS approach for the extraction of chlorinated compounds from soil samples. Talanta 81(1–2):385–391
Zhao L, Stevens J (2010) Determination of quinolone antibiotics in bovine liver using Agilent SampliQ QuEChERS kits by LC/MS/MS. Agilent Technologies, Inc. 2010 5990-5085EN
González-Curbelo MÁ, Hernández-Borges J, Ravelo-Pérez LM, Rodríguez-Delgado MÁ (2010) Insecticides extraction from banana leaves using a modified QuEChERS method. Food Chem 125(3):1083–1090
Fu RJ, Zhai A (2010) Determination of Hormones in Shrimp by Agilent 1290 Infinity LC, Poroshell 120 LC Column and QuEChERS Sample Prep. LC GC Eur: 11–12
Hernando MD, Mezcua M, Gomez MJ, Malato O, Aguera A, Fernandez-Alba AR (2004) Comparative study of analytical methods involving gas chromatography-mass spectrometry after derivatization and gas chromatography-tandem mass spectrometry for the determination of selected endocrine disrupting compounds in wastewaters. J Chromatogr A 1047(1):129–135
Xu J, Wu L, Chen W, Chang AC (2008) Simultaneous determination of pharmaceuticals, endocrine disrupting compounds and hormone in soils by gas chromatography-mass spectrometry. J Chromatogr A 1202(2):189–195
Bowers LD, Sanaullah (1996) Direct measurement of steroid sulfate and glucuronide conjugates with high-performance liquid chromatography-mass spectrometry. J Chromatogr B Biomed Sci Appl 687(1):61–68
Mansilha C, Melo A, Rebelo H, Ferreira I, Pinho O, Domingues V, Pinho C, Gameiro P (2010) Quantification of endocrine disruptors and pesticides in water by gas chromatography-tandem mass spectrometry. Method validation using weighted linear regression schemes. J Chromatogr A 1217:6681–6691
Harvey CN, Esmail M, Wang Q, Brooks AI, Zachow R, Uzumcu M (2009) Effect of the methoxychlor metabolite HPTE on the rat ovarian granulosa cell transcriptome in vitro. Toxicol Sci 110(1):95–106
Sierra-Santoyo A, Barton HA, Hughes MF (2004) Liquid chromatography determination of the anti-androgen vinclozolin and its metabolites in rat serum. J Chromatogr B 809(1):105–110
ICH (2005) International Conference on Harmonisation of technical requirements for registration of pharmaceuticals for human use. Paper presented at the ICH harmonised tripartite guideline, validation of analytical procedures: text and methodology Q2(R1), ICH Geneva
Majors RE (2008) QuEChERS—a new technique for multi-residue analysis of pesticides in foods and agricultural samples. Lc Gc Asia Pac 11(1)
EN 15662: 2009-01-01-Foods of plant origin—Determination of pesticide residues using GC-MS and/or LC-MS/MS following acetonitrile extraction/partitioning and clean-up by dispersive SPE-QuEChERS method (2009). Austrian Standards Institute,Österreichisches Normungsinstitut (ON) Heinestraße 38, 1020 Wien,
Agilent T (2009) Agilient SampliQ QuEChERS Kits. Agilent Technologies, Inc 2009 5990-3562EN
Przybylski C, Segard C (2009) Method for routine screening of pesticides and metabolites in meat based baby-food using extraction and gas chromatography-mass spectrometry. J Sep Sci 32(11):1858–1867
Borts DJ, Bowers LD (2000) Direct measurement of urinary testosterone and epitestosterone conjugates using high-performance liquid chromatography/tandem mass spectrometry. J Mass Spectrom 35(1):50–61
Annesley TM (2003) Ion suppression in mass spectrometry. Clin Chem 49(7):1041–1044. doi:10.1373/49.7.1041
Hewavitharana AK (2011) Matrix matching in liquid chromatography–mass spectrometry with stable isotope labelled internal standards—is it necessary? J Chromatogr A 1218:359–361
McNamara KM, Harwood DT, Simanainen U, Walters KA, Jimenez M, Handelsman DJ (2010) Measurement of sex steroids in murine blood and reproductive tissues by liquid chromatography-tandem mass spectrometry. J Steroid Biochem Mol Biol 121(3–5):611–618
Resko JA, Feder HH, Goy RW (1968) Androgen concentrations in plasma and testis of developing rats. J Endocrinol 40(4):485–491. doi:10.1677/joe.0.0400485
Corpechot C, Baulieu E-E, Robel P (1981) Testosterone, dihydrotestosterone and androstanediols in plasma, testes and prostates of rats during development. Acta Endocrinol 96(1):127–135. doi:10.1530/acta.0.0960127
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pouech, C., Tournier, M., Quignot, N. et al. Multi-residue analysis of free and conjugated hormones and endocrine disruptors in rat testis by QuEChERS-based extraction and LC-MS/MS. Anal Bioanal Chem 402, 2777–2788 (2012). https://doi.org/10.1007/s00216-012-5723-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-012-5723-2