Abstract
Recombinant human follicle stimulating hormone is an important drug in reproductive medicine. Thorough analysis of the heterodimeric heavily glycosylated protein is a prerequisite for the evaluation of production batches as well as for the determination of “essential similarity” of new biosimilars. The concerted application of different liquid chromatography-mass spectrometry methods enabled the complete depiction of the primary structure of this pituitary hormone. Sequence coverage of 100% for the α- as well as the β-chain was achieved with tryptic peptides. Most of these peptides could be verified by tandem mass spectrometry. Site-specific analysis of all four glycosylation sites was, however, not possible with tryptic but with chymotryptic peptides. Quantification of the glycoforms of each glycopeptide was accomplished with the software MassMap®. Both protein subunits gave interpretable mass spectra upon S-alkylation and separation on a C5 reversed-phase column. Glycan isomer patterns were depicted by separation on porous graphitic carbon, using mass spectrometric detection for the evaluation of the glycopeptide liquid chromatography-electrospray ionization data. The currently marketed product Gonal-f™ and a potential biosimilar were compared with the help of these procedures.
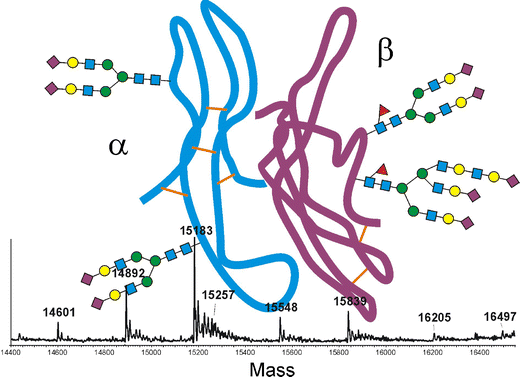
Schematic depiction of the glycoprotein nature of human follicle-stimulating hormone with the alfa chain in blue and the beta chain in purple and a mass spectrum of the alfa chain at the bottom.




Similar content being viewed by others
References
Messinis IE, Messini CI, Dafopoulos K (2010) The role of gonadotropins in the follicular phase. Ann NY Acad Sci 1205(1):5–11
Check JH (2007) Treatment of male infertility. Clin Exp Obstet Gynecol 34(4):201–206
Pierce JG, Parsons TF (1981) Glycoprotein hormones: structure and function. Annu Rev Biochem 50:465–495
Green ED, Baenziger JU (1988) Asparagine-linked oligosaccharides on lutropin, follitropin, and thyrotropin. II. Distributions of sulfated and sialylated oligosaccharides on bovine, ovine, and human pituitary glycoprotein hormones. J Biol Chem 263(1):36–44
Green ED, Baenziger JU (1988) Asparagine-linked oligosaccharides on lutropin, follitropin, and thyrotropin. I. Structural elucidation of the sulfated and sialylated oligosaccharides on bovine, ovine, and human pituitary glycoprotein hormones. J Biol Chem 263(1):25–35
Hard K, Mekking A, Damm JB, Kamerling JP, de Boer W, Wijnands RA et al (1990) Isolation and structure determination of the intact sialylated N-linked carbohydrate chains of recombinant human follitropin expressed in Chinese hamster ovary cells. Eur J Biochem 193(1):263–271
Dalpathado DS, Irungu J, Go EP, Butnev VY, Norton K, Bousfield GR et al (2006) Comparative glycomics of the glycoprotein follicle stimulating hormone: glycopeptide analysis of isolates from two mammalian species. Biochemistry 45(28):8665–8673
Stockell Hartree A, Renwick AG (1992) Molecular structures of glycoprotein hormones and functions of their carbohydrate components. Biochem J 287(Pt 3):665–679
Amoresano A, Siciliano R, Orru S, Napoleoni R, Altarocca V, De Luca E et al (1996) Structural characterisation of human recombinant glycohormones follitropin, lutropin and choriogonadotropin expressed in Chinese hamster ovary cells. Eur J Biochem 242(3):608–618
Gervais A, Hammel YA, Pelloux S, Lepage P, Baer G, Carte N et al (2003) Glycosylation of human recombinant gonadotrophins: characterization and batch-to-batch consistency. Glycobiology 13(3):179–189
Loureiro RF, de Oliveira JE, Torjesen PA, Bartolini P, Ribela MT (2006) Analysis of intact human follicle-stimulating hormone preparations by reversed-phase high-performance liquid chromatography. J Chromatogr A 1136(1):10–18
Simpson RJ (2002) Proteins and proteomics: a laboratory manual. CSHL Press, Woodbury, NY
Pabst M, Bondili JS, Stadlmann J, Mach L, Altmann F (2007) Mass + retention time = structure: a strategy for the analysis of N-glycans by carbon LC-ESI-MS and its application to fibrin N-glycans. Anal Chem 79(13):5051–5057
Packer NH, Lawson MA, Jardine DR, Redmond JW (1998) A general approach to desalting oligosaccharides released from glycoproteins. Glycoconj J 15(8):737–747
Toegel S, Pabst M, Wu SQ, Grass J, Goldring MB, Chiari C et al (2010) Phenotype-related differential alpha-2,6- or alpha-2,3-sialylation of glycoprotein N-glycans in human chondrocytes. Osteoarthritis Cartilage 18(2):240–248
Carvalho CM, Oliveira JE, Almeida BE, Ueda EK, Torjesen PA, Bartolini P et al (2009) Efficient isolation of the subunits of recombinant and pituitary glycoprotein hormones. J Chromatogr A 1216(9):1431–1438
Hokke CH, Bergwerff AA, Van Dedem GW, Kamerling JP, Vliegenthart JF (1995) Structural analysis of the sialylated N- and O-linked carbohydrate chains of recombinant human erythropoietin expressed in Chinese hamster ovary cells. Sialylation patterns and branch location of dimeric N-acetyllactosamine units. Eur J Biochem 228(3):981–1008
Stadlmann J, Pabst M, Kolarich D, Kunert R, Altmann F (2008) Analysis of immunoglobulin glycosylation by LC-ESI-MS of glycopeptides and oligosaccharides. Proteomics 8(14):2858–2871
Kolarich D, Weber A, Turecek PL, Schwarz HP, Altmann F (2006) Comprehensive glyco-proteomic analysis of human alpha1-antitrypsin and its charge isoforms. Proteomics 6(11):3369–3380
Pabst M, Altmann F (2008) Influence of electrosorption, solvent, temperature, and ion polarity on the performance of LC-ESI-MS using graphitic carbon for acidic oligosaccharides. Anal Chem 80(19):7534–7542
Melmer M, Stangler T, Schiefermeier M, Brunner W, Toll H, Rupprechter A et al (2010) HILIC analysis of fluorescence-labeled N-glycans from recombinant biopharmaceuticals. Anal Bioanal Chem 398(2):905–914
Ruhaak LR, Huhn C, Waterreus WJ, de Boer AR, Neususs C, Hokke CH et al (2008) Hydrophilic interaction chromatography-based high-throughput sample preparation method for N-glycan analysis from total human plasma glycoproteins. Anal Chem 80(15):6119–6126
Morelle W, Faid V, Chirat F, Michalski JC (2009) Analysis of N- and O-linked glycans from glycoproteins using MALDI-TOF mass spectrometry. Methods Mol Biol 534:5–21
Pabst M, Altmann F (2010) Instrumental strategies towards glycan analysis. Proteomics 11(4):631–643
Acknowledgments
The authors gratefully acknowledge Dr. Richard Peck (Basel, Switzerland) for carefully reading the manuscript and Dr. Thomas Hemetsberger for valuable advice.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in the special issue Analytical Sciences in Austria with Guest Editors G. Allmaier, W. Buchberger, and K. Francesconi.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
Analysis of recombinant human follicle-stimulating hormone (FSH) by mass spectrometric approaches (PDF 517 kb)
Rights and permissions
About this article
Cite this article
Grass, J., Pabst, M., Chang, M. et al. Analysis of recombinant human follicle-stimulating hormone (FSH) by mass spectrometric approaches. Anal Bioanal Chem 400, 2427–2438 (2011). https://doi.org/10.1007/s00216-011-4923-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-011-4923-5