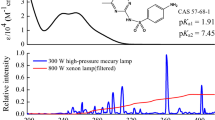
Abstract
The environmental interest of sulfonylurea herbicides was derived from the possibility of diffusion and penetration of these herbicides in the deepest layers of the ground, in particular in sandy or clay-poor soils, up to the ground waters; another interest of the study is their natural degradation pathway which leads to the formation of new species that are potentially more toxic and stable than the precursor herbicides. In this case, a lower persistence in the environment unfortunately does not correspond to a lower toxicity: hence, the importance of the identification of the species can be potentially formed. Here, nicosulfuron, a typical sulfonylurea herbicide, is considered in order to outline the environmental fate of the molecules generating from the simulation of one of the natural processes that can occur, i.e. photoinduced degradation. Aqueous nicosolfuron solutions underwent a simulated sun irradiation: the new species formed during the degradation process were identified by HPLC-DAD-MS/MS and a degradation pathway was proposed. The effect of temperature and the contribution of the hydrolysis were also evaluated. The use of ESI in both positive ion (PI) and negative ion (NI) mode and APCI in PI mode permits to obtain integrated information about the transformation products that can form; moreover, a study of the total ion chromatogram followed by the extraction of the SIM chromatograms of the most intense m/z signals made possible the identification of five possible photodegradation transformation products.







Similar content being viewed by others
References
Sarmah AK, Sabadie J (2002) Hydrolysis of sulfonylurea herbicides in soils and aqueous solutions: a review. J Agric Food Chem 50:6253–6265
Andersen SM, Hertz PB, Holst T, Bossi R, Jacobsen CS (2001) Mineralisation studies of 14C-labelled metsulfuron-methyl, tribenuron-methyl, chlorsulfuron and thifensulfuron-methyl in one Danish soil and groundwater sediment profile. Chemosphere 45:775–782
Martins JMF, Mermoud A (1999) Transport of rimsulfuron and its metabolite in soil columns. Chemosphere 38:601–616
Metzger LOY, Munier-Lamy C, Choné T, Belgy M-J, Andreux F, Védy J-C (1996) Fate of a sulfonylurea herbicide in an alluvional soil, as shown by experimental degradation of pyrimidine-2-14C-labeled rimsulfuron. Chemosphere 33:625–633
Livanainen E, Heinonen-Tanski H (1991) Degradation and leaching of chlorsulfuron in three different soils. Acta Agric Scand 41:85–92
Martins JMF, Chevre N, Spack L, Tarradellas J, Mermoud A (2001) Degradation in soil and water and ecotoxicity of rimsulfuron and its metabolites. Chemosphere 45:515–522
Olmedo C, Deban L, Coca M, Vega M, de la Rosa F (1997) Electrochemical Study of the Herbicide Tribenuron. Fresenius J Anal Chem 31:253–260
Perreau F, Bados P, Kerhoas L, Nélieu S, Einhorn J (2007) Trace analysis of sulfonylurea herbicides and their metabolites in water using a combination of off-line or on-line solid-phase extraction and liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem 388:1265–1273
Wei L, Yu H, Sun Y, Fen J, Wang L (1998) The effects of three sulfonylurea herbicides and their degradation products on the green algae, Chlorella pyrenoidosa. Chemosphere 37:747–751
Saha S, Kulshrestha G (2002) Degradation of sulfosulfuron, a sulfonylurea herbicide, as influenced by abiotic factors. J Agric Food Chem 50:4572–4575
Bossi R, Vejrup K, Jacobsen CS (1999) Determination of sulfonylurea degradation products in soil by liquid chromatography-ultraviolet detection followed by confirmatory liquid chromatography-tandem mass spectrometry. J Chromatogr A 855:575–582
Lee JK, Führ F, Kwon JW, Ahn KC (2002) Long-term fate of the herbicide cinosulfuron in lysimeters planted with rice over four consecutive years. Chemosphere 49:173–181
Khan MN, Bakar BB, Yin FWN (1999) Kinetic study on acid-base catalyzed hydrolysis of azimsulfuron, a sulfonylurea herbicide. Int J Chem Kinet 31:253–260
Shalaby LM, Bramble FQ Jr, Lee PW (1992) Application of thermospray LC/MS for residue analysis of sulfonylurea herbicides and their degradation products. J Agric Food Chem 40:513–517
Polati S, Bottaro M, Frascarolo P, Gosetti F, Mazzucco E, Gianotti V, Pollici E, Piacentini L, Pavese G, Gennaro MC (2008) HPLC-DAD-MS/MS study of tribenuron methyl photodegradation in surface waters. J Am Soc Mass Spectrom 19:1221–1229
ter Halle A, Lavieille D, Richard C (2010) The effect of mixing two herbicides mesotrione and nicosulfuron on their photochemical reactivity on cuticular wax film. Chemosphere 79:482–487
Vulliet E, Emmelin C, Chovelon JM, Guillard C, Herrmann JM (2002) Photocatalytic degradation of sulfonylurea herbicides in aqueous TiO2. Appl Catal B 38:127–137
Vulliet E, Chovelon JM, Guillard C, Hermann JM (2003) Factors influencing the photocatalytic degradation of sulfonylurea herbicides by TiO2 aqueous suspension. J Photochem Photobiol 159:71–79
Maurino V, Minero C, Pelizzetti E, Vincenti M (1999) Photocatalytic transformation of sulfonylurea herbicides over irradiated titanium dioxide particles. Colloids Surf A 151:329–338
Sleiman M, Ferronato C, Fenet B, Baudot R, Jaber F, Chovelon JM (2006) Development of HPLC/ESI-MS and HPLC/1H NMR methods for the identification of photocatalytic degradation products of iodosulfuron. Anal Chem 78:2957–2966
Polati S, Bottaro M, Frascarolo P, Gosetti F, Gianotti V, Gennaro MC (2006) HPLC-UV and HPLC-MSn multiresidue determination of amidosulfuron, azimsulfuron, nicosulfuron, rimsulfuron, thifensulfuron methyl, tribenuron methyl, and azoxystrobin in surface waters. Anal Chim Acta 579:146–151
Normative ISO 6060, (1989) Water quality determination of chemical oxygen demand
IRSA Method n. 8030 (2003) In: Metodi analitici per le acque, APAT, Rome
D.Lgs 152/2006 “Norme in materia ambientale” from the Italian Legislation in accordance with the European Directive 2000/60/CE
The e-pesticide manual, version 3.2 2005-06, 13th edn. CDS Tomlin (ed). BCPC, Hampshire
Perreau F, Bados P, Kerhoas L, Nelieu S, Einhorn J (2007) Trace analysis of sulfonylurea herbicides and their metabolites in water using a combination of off-line or on-line solid-phase extraction and liquid chromatography-tandem mass spectrometry. Anal Bional Chem 388:1265–1273
Gosetti F, Bottaro M, Gianotti V, Mazzucco E, Frascarolo P, Zampieri D, Oliveri C, Viarengo A, Gennaro MC (2010) Sun light degradation of 4-chloroaniline in waters and its effect on toxicity. a high performance liquid chromatography—diode array—tandem mass spectrometry study. Environ Pollut 158:592–598
Gianotti V, Gosetti F, Polati S, Gennaro MC (2007) HPLC-MSn and GC-MS methods to study sun and UV-lamp degradation of 1-amino-5-naphthalene sulfonate. Chemosphere 67:1993–1999
Gosetti F, Frascarolo P, Mazzucco E, Gianotti V, Bottaro M, Gennaro MC (2008) Photodegradation of E110 and E122 dyes in a commercial aperitif. A high performance liquid chromatography—diode array—tandem mass spectrometry study. J Chromatogr A 1202:58–63
Acknowledgments
The authors are grateful for the financial support from AATF (Associazione Ambiente-Territorio e Formazione, Alessandria, Italy), from Regione Piemonte, Direzione Igiene e Sanità Pubblica (Turin, Italy) and from MIUR (Ministero Italiano Università e Ricerca, Rome, Italy).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary materials
Below is the link to the electronic supplementary material.
ESM
(DOC 51 kb)
Rights and permissions
About this article
Cite this article
Benzi, M., Robotti, E. & Gianotti, V. HPLC-DAD-MSn to investigate the photodegradation pathway of nicosulfuron in aqueous solution. Anal Bioanal Chem 399, 1705–1714 (2011). https://doi.org/10.1007/s00216-010-4467-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-010-4467-0