Abstract
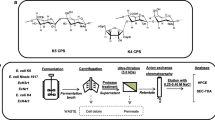
Heparosan is the key precursor for the preparation of bioengineered heparin, a potential replacement for porcine intestinal heparin, an important anticoagulant drug. The molecular weight (MW) distribution of heparosan produced by the fermentation of E. coli K5 was investigated. Large-slab isocratic and mini-slab gradient polyacrylamide gel electrophoresis (PAGE) were used to analyze the MW and polydispersity of heparosan. A preparative method that allowed fractionation by continuous-elution PAGE was used to obtain heparosan MW standards. The MWs of the heparosan standards were determined by electrospray ionization Fourier-transform mass spectrometry (ESI-FT-MS). A ladder of the standards was then used to determine the MW properties of polydisperse heparosan samples. Unbleached and bleached heparosan produced by fermentation of E. coli K5 had similar number-averaged MWs (MN), weight-averaged MWs (MW), and MW ranges of 3,000 to 150,000 Da.





Similar content being viewed by others
References
Capila I, Linhardt RJ (2002) Heparin-protein interactions. Angew Chem Int Ed Engl 41:391–412
Munoz EM, Linhardt RJ (2004) Heparin-binding domains in vascular biology. Arterioscler Thromb Vasc Biol 24:1549–1557
Raman R, Sasisekharan V, Sasisekharan R (2005) Structural insights into biological roles of protein–glycosaminoglycan interactions. Chem Biol 12:267–277
Linhardt RJ, Claude S (2003) Hudson Award address in carbohydrate chemistry. Heparin: structure and activity. J Med Chem 46:2551–2564
Kishimoto TK, Viswanathan K, Ganguly T, Elankumaran S, Smith S, Pelzer K, Lansing JC, Sriranganathan N, Zhao GL, Galcheva-Gargova Z, Al-Hakim A, Bailey GS, Fraser B, Roy S, Rogers-Cotrone T, Buhse L, Whary M, Fox J, Nasr M, Dal Pan GJ, Shriver Z, Langer R, Venkataraman G, Austen KF, Woodcock J, Sasisekharan R (2008) Contaminated heparin associated with adverse clinical events and activation of the contact system. N Engl J Med 358:2457–2467
Guerrini M, Beccati D, Shriver Z, Naggi A, Viswanathan K, Bisio A, Capila I, Lansing JC, Guglieri S, Fraser B, Al-Hakim A, Gunay NS, Zhang Z, Robinson L, Buhse L, Nasr M, Woodcock J, Langer R, Venkataraman G, Linhardt RJ, Casu B, Torri G, Sasisekharan R (2008) Oversulfated chondroitin sulfate is a contaminant in heparin associated with adverse clinical events. Nat Biotechnol 26:669–675
Guerrini M, Zhang Z, Shriver Z, Masuko S, Langer R, Casu B, Linhardt RJ, Torri G, Sasisekharan R (2009) Orthogonal analytical approaches to detect potential contaminants in heparin. Proc Natl Acad Sci USA 106:16956–16961
Zhang Z, Li B, Suwan J, Zhang F, Wang Z, Liu H, Mulloy B, Linhardt RJ (2009) Analysis of pharmaceutical heparins and potential contaminants using (1)H-NMR and PAGE. J Pharm Sci 98:4017–4026
Rudd TR, Skidmore MA, Guimond SE, Holman J, Turnbull JE, Lauder RM, Fernig DG, Yates EA (2009) The potential for circular dichroism as an additional facile and sensitive method of monitoring low-molecular-weight heparins and heparinoids. Thromb Haemost 102:874–878
Liu H, Zhang Z, Linhardt RJ (2009) Lessons learned from the contamination of heparin. Nat Prod Rep 26:313–321
Toschi V, Lettino M (2007) Fondaparinux: pharmacology and clinical experience in cardiovascular medicine. Mini Rev Med Chem 7:383–387
Zhang Z, Mccallum SA, Xie J, Nieto L, Corzana F, Jimenez-Barbero J, Chen M, Liu J, Linhardt RJ (2008) Solution structures of chemoenzymatically synthesized heparin and its precursors. J Am Chem Soc 130:12998–3007
Lindahl U, Li JP, Kusche-Gullberg M, Salmivirta M, Alaranta S, Veromaa T, Emeis J, Roberts I, Taylor C, Oreste R, Zoppetti G, Naggi A, Torri G, Casu B (2005) Generation of “neoheparin” from E. coli K5 capsular polysaccharide. J Med Chem 48:349–352
Peterson S, Frick A, Liu J (2009) Design of biologically active heparan sulfate and heparin using an enzyme-based approach. Nat Prod Rep 26:610–627
Kuberan B, Beeler DL, Lech M, Wu ZL, Rosenberg RD (2003) Chemoenzymatic synthesis of classical and non-classical anticoagulant heparan sulfate polysacchrides. J Biol Chem 278:52613–52621
Blundell CD, Roberts IS, Sheehan JK, Almond A (2009) Investigating the molecular basis for the virulence of Escherichia coli K5 by nuclear magnetic resonance analysis of the capsule polysaccharide. J Mol Microbiol Biotechnol 17:71–82
Manzoni M, Bergomi S, Cavazzoni V (1993) Extracellular K5 polysaccharide of Escherichia coli: production and characterization. J Bioact Compat Polym 8:251–257
Manzoni M, Bergomi S, Rollini M (2000) Influence of the culture conditions on extracellular lyase activity related to K5 polysaccharide. Biotechnol Lett 22:81–85
Volpi N (2004) Purification of the Escherichia coli K5 capsular polysaccharide and use of high-performance capillary electrophoresis to qualitative and quantitative monitor the process. Electrophoresis 25:3307–3312
Vann WF, Schmidt MA, Jann B, Jann K (1981) The structure of the capsular polysaccharide (K5 antigen) of urinary-tract-infective Escherichia coli 010:K5:H4. A polymer similar to desulfo-heparin. Eur J Biochem 116:359–364
Sigulinsky C, Babu P, Victor XV, Kuberan B (2010) Preparation and characterization of (15)N-enriched, size-defined heparan sulfate precursor oligosaccharides. Carbohydr Res 345:250–256
Laremore TN, Ly M, Solakyildirim K, Zagorevski DV, Linhardt RJ (2010) High-resolution preparative separation of glycosaminoglycan oligosaccharides by polyacrylamide gel electrophoresis. Anal Biochem doi:10.1016/j.ab.2010.03.004 (in press)
Edens RE, Al-Hakim A, Weiler JM, Rethwisch DG, Fareed J, Linhardt RJ (1992) Gradient polyacrylamide gel electrophoresis for determination of molecular weights of heparin preparations and low-molecular-weight heparin derivatives. J Pharm Sci 81:823–827
Grant AC, Linhardt RJ, Fitzgerald GL, Park JJ, Langer R (1984) Metachromatic activity of heparin and heparin fragments. Anal Biochem 137:25–32
Acknowledgements
The authors are grateful to the National Institutes of Health for supporting this research with grants GM38060 and HL096972.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 682 kb)
Rights and permissions
About this article
Cite this article
Ly, M., Wang, Z., Laremore, T.N. et al. Analysis of E. coli K5 capsular polysaccharide heparosan. Anal Bioanal Chem 399, 737–745 (2011). https://doi.org/10.1007/s00216-010-3679-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-010-3679-7