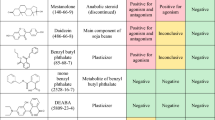
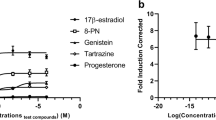
Abstract
In vitro assays are considered as the first step in a tiered approach to compound screening for hormonal activity. Although many new assays have been developed in recent years, little attention has been paid towards assay validation. Our objective was to identify critical experimental parameters in a yeast estrogen screen (YES) that affect its sensitivity and specificity. We investigated the role of incubation time, solvent type, yeast inoculum growth stage and concentration on the outcome of the YES. Compounds tested included new and established agonists, antagonists and negative controls, and results were evaluated according to prefixed statistical criteria. In addition, we assessed the assay’s performance in a blind interlaboratory validation exercise (IVE). An incubation time of five days was necessary to positively identify the estrogenic properties of all agonists tested, when dissolved in DMSO. Longer incubation times were required when using an ethanol protocol. Similar estrogenic activity was reported for benzyl butyl phthalate, bisphenol-A, methoxychlor, permethrin and genistein in the IVE. One out of the three laboratories did not classify α,β-endosulfan, dissolved in DMSO, as an estrogen. The same was true for 4,4′-DDE and lindane, dissolved in ethanol, a result that might be attributable to an inappropriate yeast start concentration and/or growth stage. These validation experiments show that under appropriate experimental conditions the YES yields sensitive, specific and reliable results. Therefore it fulfills the requirements as a first step screening assay to evaluate the capacity of chemicals to interact with the estrogen receptor.


Similar content being viewed by others
Abbreviations
- AU:
-
absorbance units
- BBP:
-
benzyl butyl phthalate
- CAU:
-
corrected AU
- CPRG:
-
chlorophenol red-β-D-galactopyranoside
- CI:
-
confidence interval
- CV:
-
coefficient of variation
- DBP:
-
dibutyl phthalate
- DMSO:
-
dimethylsulfoxide
- EC50 :
-
50% effect concentration
- E2:
-
17β-estradiol
- ER:
-
estrogen receptor
- E2-EQs:
-
17β-estradiol equivalents
- ERE:
-
estrogen responsive element
- GM:
-
geometric mean
- ICCVAM:
-
interagency coordinating committee on the validation of alternative methods
- IVE:
-
interlaboratory validation exercise
- LOD:
-
limit of detection
- OD:
-
optical density
- REACH:
-
registration, evaluation and authorisation of chemicals
- RIE:
-
relative induction efficiency
- RP:
-
relative potency
- SD:
-
standard deviation
- TA:
-
transactivation
- YES:
-
yeast estrogen screen
- 95%CI:
-
95% confidence interval
- 2,4-D:
-
(2,4-dichlorophenoxy)acetic acid
- 4-OHT:
-
4-hydroxytamoxifen
- 4,4′-DDT:
-
1,1,1-trichloro-2,2-bis(4-chlorophenyl)ethane
- 4,4′-DDE:
-
1,1-dichloro-2,2-bis(4-chlorophenyl)ethylene
- 4,4′-TDE:
-
1,1-dichloro-2,2-bis(4-chlorophenyl)ethane
References
The National Toxicology Program (NTP) Interagency Center for the Evaluation of Alternative Toxicological Methods (NICEATM) (2002) Background review document (BRD) current status of test methods for detecting endocrine disruptors: in vitro estrogen receptor transcriptional activation assays National Institute of Environmental Health Sciences (NIEHS). NIH Publication No. 03-4505. 2002. Research Triangle Park, NC 27709
Crisp TM, Clegg ED, Cooper RL, Wood WP, Anderson DG, Baetcke KP, Hoffmann JL, Morrow MS, Rodier DJ, Schaeffer JE, Touart LW, Zeeman MG, Patel YM (1998) Environ Health Perspect 106 (Suppl 1):11–56
Gray LE, Ostby J, Furr J, Wolf CJ, Lambright C, Parks L, Veeramachaneni DN, Wilson V, Price M, Hotchkiss A, Orlando E, Guillette L (2001) Hum Reprod Update 7:248–264
Sharpe RM, Irvine DS (2004) BMJ 328:447–451
European Commission. REACH: Commission welcomes Council’s agreement on new EU chemical legislation (ref IP/05/1583). European Commission Press Releases 2005
Gray LE, Ostby J, Wilson V, Lambright C, Bobseine K, Hartig P, Hotchkiss A, Wolf C, Furr J, Price M, Parks L, Cooper RL, Stoker TE, Laws SC, Degitz SJ, Jensen KM, Kahl MD, Korte JJ, Makynen EA, Tietge JE, Ankley GT (2002) Toxicology 181:371–382
Andersen HR, Andersson AM, Arnold SF, Autrup H, Barfoed M, Beresford NA, Bjerregaard P, Christiansen LB, Gissel B, Hummel R, Jørgensen EB, Korsgaard B, Le-Guevel R, Leffers H, McLachlan J, Møller A, Nielsen JB, Olea N, Oles-Karasko A, Pakdel F, Pedersen KL, Perez P, Skakkebaek NE, Sonnenschein C, Soto AM, Sumpter JP, Thorpe SM, Grandjean P (1999) Environ Health Perspect 107:89–108
Gaido KW, Leonard LS, Lovell S, Gould JC, Babaï D, Portier CJ, McDonnell DP (1997) Toxicol Appl Pharmacol 143:205–212
Petit F, Le Goff P, Cravédi JP, Valotaire Y, Pakdel F (1997) J Mol Endocrinol 19:321–335
Routledge EJ, Parker J, Odum J, Ashby J, Sumpter JP (1998) Toxicol Appl Pharmacol 153:12–19
Bovee TF, Helsdingen RJ, Rietjens IM, Keijer J, Hoogenboom RL (2004) J Steroid Biochem Mol Biol 91:99–109
Fang H, Tong WD, Perkins R, Soto AM, Prechtl NV, Sheehan DM (2000) Environ Health Perspect 108:723–729
Ekins R, Edwards P (1997) Clin Chem 43:1824–1831
Interagency Coordinating Committee on the Validation of Alternative Methods (ICCVAM) and The National Toxicology Program (NTP) Interagency Center for the Evaluation of Alternative Toxicological Methods (NICEATM) (2003) ICCVAM evaluation of in vitro test methods for detecting potential endocrine disruptors: estrogen receptor and androgen receptor binding and transcriptional activation assays. National Institute of Environmental Health Sciences (NIEHS). NIH Publication No. 03-4503. 2003
Routledge EJ, Sumpter JP (1996) Environ Toxicol Chem 15:241–248
Beresford N, Routledge EJ, Harris CA, Sumpter JP (2000) Toxicol Appl Pharmacol 162:22–33
De Boever P, Demaré W, Vanderperren E, Cooreman K, Bossier P, Verstraete W (2001) Environ Health Perspect 109:691–697
Schultz TW, Sinks GD, Cronin MTD (2000) Environ Toxicol Chem 19:2637–2642
Soto AM, Sonnenschein C, Chung KL, Fernandez MF, Olea N, Serrano FO (1995) Environ Health Perspect 103:113–122
Lyttle CR, Damian-Matsumura P, Juul H, Butt TR (1992) J Steroid Biochem Mol Biol 42:677–685
Dudley MW, Sheeler CQ, Wang H, Khan S (2000) Proc Natl Acad Sci USA 97:3696–3701
Gray JV, Petsko GA, Johnston GC, Ringe D, Singer RA, Werner-Washburne M (2004) Microbiol Mol Biol Rev 68:187–206
Klis FM, Mol P, Hellingwerf K, Brul S (2002) FEMS Microbiol Rev 26:239–256
Mahé Y, Lemoine Y, Kuchler K (1996) J Biol Chem 271:25167–25172
Liu JW, Jeannin E, Picard D (1999) Biol Chem 380:1341–1345
Wolfger H, Mamnun YM, Kuchler K (2004) J Biol Chem 279:11593–11599
Schwarzenbach R, Gschwend PM, Imboden DM (1993) Environmental organic chemistry. Wiley
Fassel TA, Sohnle PG, Kushnaryov VM (1997) Biotech Histochem 72:268–272
Bovee TF, Helsdingen RJ, Koks PD, Kuiper HA, Hoogenboom RL, Keijer J (2004) Gene 325:187–200
Berry M, Metzger D, Chambon P (1990) EMBO J 9:2811–2818
Schultz TW, Sinks GD, Cronin MT (2002) Environ Toxicol 17:14–23
Nelson DR (1999) Arch Biochem Biophys 369:1–10
Parkinson A, Safe S (1982) Biochem Pharmacol 31:1849–1856
Elsby R, Ashby J, Sumpter JP, Brooks AN, Pennie WD, Maggs JL, Lefevre PA, Odum J, Beresford N, Paton D, Park BK (2000) Biochem Pharmacol 60:1519–1530
Coldham NG, Dave M, Sivapathasundaram S, McDonnell DP, Connor C, Sauer MJ (1997) Environ Health Perspect 105:734–742
Black JW, Leff P (1983) Proc R Soc Lond B Biol Sci 220:141–162
Jordan VC (2003) J Med Chem 46:883–908
Shang Y, Brown M (2002) Science 295:2465–2468
Cheskis BJ, Karathanasis S, Lyttle CR (1997) J Biol Chem 272:11384–11391
Legler J, Dennekamp M, Vethaak AD, Brouwer A, Koeman JH, van der BB, Murk AJ (2002) Sci Total Environ 293:69–83
Tyler CR, Beresford N, van der Woning M, Sumpter JP, Thorpe K (2000) Environ Toxicol Chem 19:801–809
Freyberger A, Schmuck G (2005) Toxicol Lett 155:1–13
Körner W, Vinggaard AM, Térouanne B, Ma R, Wieloch C, Schlumpf M, Sultan C, Soto AM (2004) Environ Health Perspect 112:695–702
Pöch G, Reiffenstein RJ, Baer HP (1995) J Pharmacol Toxicol Methods 33:197–204
Acknowledgement
Willem Dhooge is supported by a fund from the Support Group Environment and Health, financed by the Flemish Government (Department of Science, Department of Public Health and Department of Environment, Brussels, Belgium).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dhooge, W., Arijs, K., D’Haese, I. et al. Experimental parameters affecting sensitivity and specificity of a yeast assay for estrogenic compounds: results of an interlaboratory validation exercise. Anal Bioanal Chem 386, 1419–1428 (2006). https://doi.org/10.1007/s00216-006-0669-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-006-0669-x