Abstract
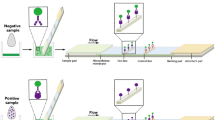
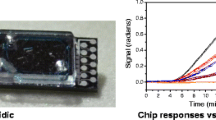
We report on recent advances of our immunoassay for the hormone progesterone in cow’s milk. Detection is based on total internal reflectance fluorescence (TIRF), the binding-inhibition assay with an immobilized progesterone derivative, and a commercially available monoclonal antibody to progesterone as biological recognition element. The fully automated River Analyzer (RIANA) biosensor for unattended, cost-effective, and continuous monitoring of environmental pollution therefore was adapted for sensitive determination of progesterone in milk. First, the sensitivity and robustness of the existing progesterone assay for water analysis were improved, resulting in a detection limit (LOD) of only 0.2 pg mL−1 and a quantification limit (LOQ) of only 2.0 pg mL−1. These extraordinary results are the lowest detection and quantification limits for progesterone determination using biosensors yet reported in the literature. Second, the accurate indicator of ovulation was calibrated and detected in three different types of milk (UHT milk, fresh milk, and raw milk). For commercial milk and randomly procured raw milk nominal levels of progesterone are typically in the range 5–15 ng mL−1. Limits of detection (LOD) achieved for added progesterone (i.e. spiked samples) were between 45.5 and 56.1 pg mL−1 depending on milk type. Having in mind the 1:10 dilution factor, these results are still a success. For the first time a commercially available antibody was incorporated into an immunoassay for progesterone detection in bovine milk, giving a detection limit below 1 ng mL−1 for a fully automated biosensor. Thus the outstanding progress made with this biosensor in environmental monitoring and water analysis has now been successfully adapted to milk analysis for use in the field of reproduction management.





Similar content being viewed by others
References
Esslemont RJ, Bryant MJ (1976) Vet Rec 99:472–475
Esslemont RJ, Eddy RG, Ellis PR (1977) Vet Rec 100:426–427
Esslemont RJ, Eddy RG (1977) Br Vet J 133:346–355
Mottram T, Velasco-Garcia M, Berry P, Richards P, Ghesquiere J, Masson L (2002) Comp Clin Pathol 11:50–58
Mottram TT, Frost AR (1997) Report on the state of the art of current and emerging methods of oestrus detection on UK dairy farms. Contract Report CR/775/97/1681
Pope GS, Swinburne JK (1980) J Dairy Res 47:427–449
Delwiche MJ, Tang X, Bondurant R (1998) On-line measurement of progesterone during milking for estrus detection. In: AgEng 98, International Conference on Agricultural Engineering, Oslo, 24–27 August Part 1, CIGR, pp 59–60
Claycomb RW, Delwiche MJ (1998) Biosens Bioelectron 13:1173–1180
Pemberton RM, Hart JP, Mottram TT (2001) Biosens Bioelectron 16:715–723
Delwiche M, Tang X, BonDurant R, Munro C (2001) Trans ASAE 44:1997–2002
Gillis EH, Gosling JP, Sreenan JM, Kane M (2002) J Immunol Methods 267:131–138
Kreuzer MP, McCarthy R, Pravda M, Guilbault GG, (2004) Anal Lett 37:943–956
Sananikone K, Delwiche MJ, BonDurant RH, Munro CJ (2004) Trans ASAE 47:1357–1365
Rodriguez-Mozaz S, Reder S, de Alda ML, Gauglitz G, Barcelo D (2004) Biosensors Bioelectron 19:633–640
Tschmelak J, Proll G, Gauglitz G (2004) Biosens Bioelectron 20:743–752
Willard D, Proll G, Reder S, Gauglitz G (2003) Environ Sci Pollut Res Int 10:188–191
Tschmelak J, Proll G, Riedt J, Kaiser J, Kraemmer P, Barzaga L, Wilkinson JS, Hua P, Hole JP, Nudd R, Jackson M, Abuknesha R, Barcelo D, Rodriguez-Mozaz S, de Alda MJL, Sacher F, Stien J, Slobodnik J, Oswald P, Kozmenko H, Korenkova E, Tothova L, Krascsenits Z, Gauglitz G (2005) Biosens Bioelectron 20:1499–1508
Tschmelak J, Proll G, Riedt J, Kaiser J, Kraemmer P, Barzaga L, Wilkinson JS, Hua P, Hole JP, Nudd R, Jackson M, Abuknesha R, Barcelo D, Rodriguez-Mozaz S, de Alda MJL, Sacher F, Stien J, Slobodnik J, Oswald P, Kozmenko H, Korenkova E, Tothova L, Krascsenits Z, Gauglitz G (2005) Biosens Bioelectron 20:1509–1519
Tschmelak J, Proll G, Gauglitz G (2005) Talanta 65:313–323
Tschmelak J, Proll G, Gauglitz G (2004) Anal Bioanal Chem 379:1004–1012
Tschmelak J, Proll G, Gauglitz G (2004) Anal Chim Acta 519:143–146
Barzen C, Brecht A, Gauglitz G (2002) Biosens Bioelectron 17:289–295
Glaser RW (1993) Anal Biochem 213:152–161
Kroger K, Jung A, Reder S, Gauglitz G (2002) Anal Chim Acta 469:37–48
Jung A, Stemmler I, Brecht A, Gauglitz G (2001) Fresenius J Anal Chem 371:128–136
Dudley RA, Edwards P, Ekins RP, Finney DJ, McKenzie IG, Raab GM, Rodbard D, Rodgers RP (1985) Clin Chem 31:1264–1271
Inczedy J, Lengyel T, Ure AM (1998) Compendium of analytical nomenclature—the orange book, 3rd edn. Blackwell Science, Oxford
O’Connell MA, Belanger BA, Haaland PD (1993) Chemometr Intell Lab Sys 20:97–114
Kavlock RJ, Daston GP, DeRosa C, Fenner-Crisp P, Gray LE, Kaattari S, Lucier G, Luster M, Mac MJ, Maczka C, Miller R, Moore J, Rolland R, Scott G, Sheehan DM, Sinks T, Tilson HA (1996) Environ Health Perspect 104:715–740
Shore LS, Gurevitz M, Shemesh M (1993) Bull Environ Contam Toxicol 51:361–366
Acknowledgements
This work was funded by the “Automated Water Analyzer Computer Supported System” (AWACSS) (EVK1-CT-2000-00045) research project supported by the European Commission under the Fifth Framework Program and contributing to the implementation of the Key Action “Sustainable Management and Quality of Water” within the Energy, Environment and Sustainable Development Program. The development of the biosensor used was funded by the European Commission under the Environment and Climate Program (Fourth Framework Program) River Analyzer (RIANA) (ENV4-CT95-0066) project. The derivative progesterone-11-hemisuccinate for the surface chemistry was kindly supplied by Ram Abuknesha, King’s College London, London, UK. Jens Tschmelak is a scholarship holder of the research training group “Quantitative Analysis and Characterization of Pharmaceutically and Biochemically relevant Substances” funded by the Deutsche Forschungsgemeinschaft (DFG) at the Eberhard-Karls-University of Tuebingen, Germany.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to the memory of Wilhelm Fresenius.
Rights and permissions
About this article
Cite this article
Tschmelak, J., Käppel, N. & Gauglitz, G. TIRF-based biosensor for sensitive detection of progesterone in milk based on ultra-sensitive progesterone detection in water. Anal Bioanal Chem 382, 1895–1903 (2005). https://doi.org/10.1007/s00216-005-3261-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-005-3261-x