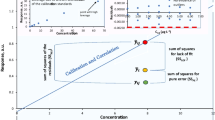
Abstract
A critical discussion about the possibility of improving the method validation process by means of experimental design is presented. The reported multivariate strategies concern the evaluation of the performance parameters robustness and intermediate precision, and the optimisation of bias and repeatability. In particular, accuracy and precision improvement constitutes a special subset of experimental design in which the bias and the relative standard deviation of the assay are optimised. D-optimal design was used in order to plan experiments for this aim. The analytical methods considered were capillary electrophoresis, HPLC, adsorptive stripping voltammetry and differential pulse polarography. All methods were applied to real pharmaceutical analysis problems.




Similar content being viewed by others
References
Green JM (1996) Anal Chem 68:305A-309A
Thomposon M, Ellison S, Wood R (2002) Pure Appl Chem 74:835–855
ICH Harmonised tripartite guideline Q2B (1996) Validation of Analytical Procedures: Methodology. ICH, Geneva
The United States Pharmacopeia (2003) USP26, The United States Pharmacopeia, USA, pp. 2439–2443
Swartz ME, Krull I (1997) Analytical method development and validation. Dekker, New York
Lewis GA, Mathieu D, Phan-Tan-Luu R (1999) Pharmaceutical experimental design. Dekker, New York
Montgomery DC (1997) Design and analysis of experiments. Wiley, New York
Carlson R (1992) Design and optimization in organic synthesis. Elsevier, Amsterdam
Pieniaszek HJ (1999) Short course on bioanalytical method development, validation and transfer. 10th International Symposium on Pharmaceutical and Biomedical Analysis, Washington, D.C., USA, May 9. Short course material
Chen JG, Glancy K, Chen X, Alessandro M (2001) J Chromatogr A 917:63–73
Achilli M, Romele L (1997) J Chromatogr A 770:29–37
Mathieu D, Phan-Tan-Luu R, Sergent M (1998) Méthodes modernes d'élaboration de matrices d'expériences optimales et qualité. LPRAI, Marseille
Box G, Meyer D (1986) Technometrics 28:19–26
Polaskova P, Bocaz G, Li H, Havel J (2002) J Chromatogr A 979:59–67
Furlanetto S, Maestrelli F, Orlandini S, Pinzauti S, Mura P (2003) J Pharm Biomed Anal 32:159–165
Hund E, Vander Heyden Y, Haustein M, Massart DL, Smeyers-Verbeke J (2000) Anal Chim Acta 404:257–271
Fabre H (1996) J Pharm Biomed Anal 14:1125–1132
Vander Heyden Y, Hartmann C, Massart DL, Michel L, Kiechle P, Erni F (1995) Anal Chim Acta 316:15–26
Ragonese R, Mulholland M, Kalman J (2000) J Chromatogr A 870:45–51
Owens P, Wikstrom H, Nagard S, Karlsson L (2002) J Pharm Biomed Anal 27:587–598
Furlanetto S, Pinzauti S, La Porta E, Chiarugi A, Mura P, Orlandini S (1998) J Pharm Biomed Anal 17:1015–1028
Furlanetto S, Pinzauti S, Gratteri P, La Porta E, Calzeroni G (1997) J Pharm Biomed Anal 15:1585–1594
Furlanetto S, Orlandini S, La Porta E, Coran S, Pinzauti S (2002) J Pharm Biomed Anal 28:1161–1171
Gotti R, Furlanetto S, Andrisano V, Cavrini V, Pinzauti S (2000) J Chromatogr A 875:411–422
Ficarra R, Ficarra P, Tommasini S, Melardi S, Calabrò ML, Furlanetto S, Semreen M (2000) J Pharm Biomed Anal 23:169–174
Ficarra R, Calabrò M, Cutroneo P, Tommasini S, Melardi S, Semreen M, Furlanetto S, Ficarra P, Altavilla G (2002) J Pharm Biomed Anal 29:1097–1103
Furlanetto S, Orlandini S, Massolini G, Faucci M, La Porta E, Pinzauti S (2001) Analyst 126:1700–1706
Altria K, Bryant S, Hadgett T (1997) J Pharm Biomed Anal 15:1091–1101
Altria K (1996) J Chromatogr A 735:43–56
Altria K, Clark B, Filbey S, Kelly M, Rudd D (1995) Electrophoresis 16:2143–2148
Altria K, Filbey S (1994) Chromatographia 39:306–310
Furlanetto S, Tognini C, Carpenedo R, La Porta E, Pinzauti S (1998) J Pharm Biomed Anal 18:67–73
Furlanetto S, Orlandini S, Aldini G, Gotti R, Dreassi E, Pinzauti S (2000) Anal Chim Acta 413:229–239
Ye C, Liu J, Ren F, Okafo N (2000) J Pharm Biomed Anal 23:581–589
Mathieu D, Nony J, Phan-Tan-Luu R (2000) NEMROD-W. LPRAI, Marseille
Kaale E, Van Schepdael A, Roets E, Hoogmartens J (2001) J Chromatogr A 924:451–458
Marchesini A, Williner M, Mantovani V, Robles J, Goicoechea H (2003) J Pharm Biomed Anal 31:39–46
Stalberg O, Sander K, Sanger-van de Griend C (2002) J Chromatogr A 977:265–275
Ficarra R, Cutroneo P, Aturki Z, Tommasini S, Calabrò M, Phan-Tan-Luu R, Fanali S, Ficarra P (2002) J Pharm Biomed Anal 29:989–997
Ragonese R, Macka M, Hughes J, Petocz P (2002) J Pharm Biomed Anal 27:995–1007
Saavedra L, Barbas C (2002) J Chromatogr B 766:235–242
Akesolo U, Gonzalez L, Jimenez R, Alonso R (2003) J Chromatogr A 990:271–279
Furlanetto S, Gratteri P, Pinzauti S, Leardi R, Dreassi E, Santoni G (1995) J Pharm Biomed Anal 13:431–438
European Community (2002) Official Journal of the European Communities L 221, 17.8.2002, p. 8–36
Meier P, Zünd R (1993) Statistical methods in analytical chemistry. Wiley, New York
Frank IE, Todeschini R (1994) The data analysis handbook. Elsevier, Amsterdam
de Aguiar PF, Bourguignon B, Khots MS, Massart DL, Phan-Tan-Luu R (1995) Chemom Intell Lab Sys 30:199–210
Mathieu D, Phan-Tan-Luu R, Sergent M (1996) Criblage et étude des facteurs. LPRAI, Marseille
Lenth RV (1989) Technometrics 3:469–473
Vander Heyden Y, Massart DL (1996) Review of the use of robustness and ruggedness in analytical chemistry. In: Smilde A, de Boer J, Hendricks M (eds) Robustness of analytical methods and pharmaceutical technological products. Elsevier, Amsterdam pp 79–147
Vander Heyden Y, Nijhuis A, Smeyers-Verbeke J, Vandeginste BGM, Massart DL (2001) J Pharm Biomed Anal 24:723-753
van Leeuwen JA, Buydens LMC, Vandeginste BGM, Kateman G, Schoenmakers PJ, Mulholland M (1991) Chemom Intell Lab Sys 10:337–347
Plackett RL, Burman JP (1946) Biometrika 33:305
Fanali S, Furlanetto S, Aturki Z, Pinzauti S (1998) Chromatographia 48:395–401
Acknowledgements
This work was supported in part by a grant from MIUR.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Furlanetto, S., Orlandini, S., Mura, P. et al. How experimental design can improve the validation process. Studies in pharmaceutical analysis. Anal Bioanal Chem 377, 937–944 (2003). https://doi.org/10.1007/s00216-003-2189-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-003-2189-2