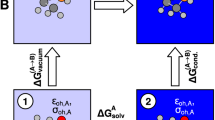
Abstract
Three quantum chemistry methods (B3LYP, M05-2X and CBS-4B3*) have been used, in combination with SMD and CPCM continuum solvent models, to calculate the aqueous pK a values of common organic compounds (aliphatic alcohols, carboxylic acids, amines, phenols, benzoic acids and pyridines) by using an isodesmic reaction. Good precision is found for all the studied functional groups, resulting mean absolute deviations of 0.5–1 pK a units (equivalent to the best results obtained with thermodynamic cycles). It is worthy to note that no explicit water molecules were needed with the isodesmic reaction. In addition, the quality of the results is not strongly dependent on the combination of quantum chemistry method, solvent model and reference species. Therefore, the isodesmic reaction could be successfully used when dealing with gas-phase unstable species, with species that undergo large conformational changes between gas-phase and solution-phase or other difficult cases for the thermodynamic cycles.


Similar content being viewed by others
References
Ho J, Coote ML (2010) A universal approach for continuum solvent pK a calculations: are we there yet? Theor Chem Acc 125:3–21
Ho J, Klamt A, Coote ML (2010) Comment on the correct use of continuum solvent models. J Phys Chem A 114:13442–13444
Casasnovas R, Fernández D, Ortega-Castro J, Frau J, Donoso J, Muñoz F (2011) Avoiding gas-phase calculations in theoretical pK a predictions. Theor Chem Acc 130:1–13
Takano Y, Houk KN (2005) Benchmarking the conductor-like polarizable continuum model (CPCM) for aqueous solvation free energies of neutral and ionic organic molecules. J Chem Theory Comput 1:70–77
Cramer CJ, Truhlar DG (2008) A universal approach to solvation modeling. Acc Chem Res 41:760–768
Wang L, Heard DE, Pilling MJ, Seakins P (2008) A Gaussian-3X prediction on the enthalpies of formation of chlorinated phenols and Dibenzo-p-dioxins. J Phys Chem A 112:1832–1840
Li GS, Ruiz-López MF, Maigret B (1997) Ab initio study of 4(5)-Methylimidazole in aqueous solution. J Phys Chem A 101:7885–7892
Derbel N, Clarot I, Mourer M, Regnouf-de-Vains J, Ruiz-López MF (2012) Intramolecular interactions versus hydration effects on p-Guanidinoethyl-phenol structure and pK a values. J Phys Chem A 116:9404–9411
Govender KK, Cukrowski I (2009) Density functional theory in prediction of four stepwise protonation constants for nitrilotripropanoic acid (NTPA). J Phys Chem A 113:3639–3647
Govender KK, Cukrowski I (2010) Density functional theory and isodesmic reaction based prediction of four stepwise protonation constants, as log K (n)H , for nitrilotriacetic acid. the importance of a kind and protonated form of a reference molecule used. J Phys Chem A 114:1868–1878
Casasnovas R, Frau J, Ortega-Castro J, Salvà A, Donoso J, Muñoz F (2009) Absolute and relative pK a calculations of mono and diprotic pyridines by quantum methods. J Mol Struct Theochem 912:5–12
Gao DQ, Svoronos P, Wong PK, Maddalena D, Hwang J, Walker H (2005) pK a of acetate in water: a computational study. J Phys Chem A 109:10776–10785
Ho J, Coote ML (2009) pK a calculation of some biologically important carbon acids—an assessment of contemporary theoretical procedures. J Chem Theory Comput 5:295–306
Barone V, Cossi M (1998) Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. J Phys Chem A 102:1995–2001
Barone V, Cossi M, Tomassi J (1998) Geometry optimization of molecular structures in solution by the polarizable continuum model. J Comput Chem 19:404–417
Cossi M, Rega M, Scalmani G, Barone V (2003) Energies, structures, and electronic properties of molecules in solution with the C-PCM solvation model. J Comput Chem 24:669–681
Marenich AV, Cramer CJ, Truhlar DG (2009) Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J Phys Chem B 113:6378–6396
Marenich AV, Cramer CJ, Truhlar DG (2009) Performance of SM6, SM8, and SMD on the SAMPL1 test set for the prediction of small-molecule solvation free energies. J Phys Chem B 113:4538–4543
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652
Zhao Y, Schultz NE, Truhlar DG (2006) Design of density functionals by combining the method of constraint satisfaction with parametrization for thermochemistry, thermochemical kinetics, and noncovalent interactions. J Chem Theory Comput 2:364–382
Zhao Y, Truhlar DG (2008) Functionals with broad applicability in chemistry. Acc Chem Res 41:157–167
Casasnovas R, Frau J, Ortega-Castro J, Salvà A, Donoso J, Muñoz F (2010) Simplification of the CBS-QB3 method for predicting gas-phase deprotonation free energies. Int J Quantum Chem 110:323–330
Gaussian 03, Revision E.01, Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery Jr JA, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick D K, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox D J, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill P MW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA, Gaussian, Inc., Wallingford CT, (2004)
Gaussian 09, Revision B.01, Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery Jr JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ, Gaussian, Inc., Wallingford CT (2009)
Pliego JR, Riveros JM (2002) Theoretical calculation of pK a using the cluster-continuum model. J Phys Chem A 106:7434–7439
Namazian M, Heidary H (2003) Ab initio calculations of pK a values of some organic acids in aqueous solution. J Mol Struct-Theochem 620:257–263
Silva CO, da Silva EC, Nascimento MAC (2000) Ab initio calculations of absolute pK a values in aqueous solution II. Aliphatic alcohols, thiols, and halogenated carboxylic acids. J Phys Chem A 104:2402–2409
Kelly CP, Cramer CJ, Truhlar DG (2006) Adding explicit solvent molecules to continuum solvent calculations for the calculation of aqueous acid dissociation constants. J Phys Chem A 110:2493–2499
Liptak MD, Shields GC (2001) Accurate pK a calculations for carboxylic acids using complete basis set and Gaussian-n models combined with CPCM continuum solvation methods. J Am Chem Soc 123:7314–7319
Liptak MD, Shields GC (2001) Experimentation with different thermodynamic cycles used for pKa calculations on carboxylic acids using complete basis set and gaussian-n models combined with CPCM continuum solvation methods. Int J Quantum Chem 85:727–741
Toth AM, Liptak MD, Phillips DL, Shields GC (2001) Accurate relative pK a calculations for carboxylic acids using complete basis set and Gaussian-n models combined with continuum solvation methods. J Chem Phys 114:4595–4606
Namazian M, Halvani S (2006) Calculations of pK a values of carboxylic acids in aqueous solution using density functional theory. J Chem Thermodyn 38:1495–1502
Namazian M, Halvani S, Noorbala MR (2004) Density functional theory response to the calculations of pK a values of some carboxylic acids in aqueous solution. J Mol Struct Theochem 711:13–18
Schuurmann G, Cossi M, Barone V, Tomasi J (1998) Prediction of the pK a of carboxylic acids using the ab initio continuum-solvation model PCM-UAHF. J Phys Chem A 102:6706–6712
da Silva CO, da Silva EC, Nascimento MAC (1999) Ab initio calculations of absolute pK a values in aqueous solution I. Carboxylic acids. J Phys Chem A 103:11194–11199
Saracino GAA, Improta R, Barone V (2003) Absolute pK a determination for carboxylic acids using density functional theory and the polarizable continuum model. Chem Phys Lett 373:411–415
Schmidt am Busch M, Knapp EW (2004) Accurate pK a determination for a heterogeneous group of organic molecules. Chem Phys Chem 5:1513–1522
Jia Z, Du D, Zhou Z, Zhang A, Hou R (2007) Accurate pK a determinations for some organic acids using an extended cluster method. Chem Phys Lett 439:374–380
da Silva EF, Svendsen HF (2003) Prediction of the pK a values of amines using ab initio methods and free-energy perturbations. Ind Eng Chem Res 42:4414–4421
Eckert F, Klamt A (2006) Accurate prediction of basicity in aqueous solution with COSMO-RS. J Comput Chem 27:11–19
Blanco SE, Ferretti FH (2005) Calculation of ionization constants of methylamines in aqueous solution. J Mol Struc Theochem 722:197–202
Behjatmanesh-Ardakani R, Karimi MA, Ebady A (2009) Cavity shape effect on pK a prediction of small amines. J Mol Struc Theochem 910:99–103
Khalili F, Henni A, East ALL (2009) Entropy contributions in pK a computation: application to alkanolamines and piperazines. J Mol Struc Theochem 916:1–9
Liptak MD, Gross KC, Seybold PG, Feldgus S, Shields GC (2002) Absolute pK a determinations for substituted phenols. J Am Chem Soc 124:6421–6427
Rebollar-Zepeda AM, Campos-Hernández T, Ramirez MT, Rojas-Hernández A, Galano A (2011) Searching for computational strategies to accurately predict pK as of large phenolic derivatives. J Chem Theory Comput 7:2528–2538
Caballero NA, Melendez FJ, Muñoz-Caro C, Niño A (2006) Theoretical prediction of relative and absolute pK a values of aminopyridines. Biophys Chem 124:155–160
Chen IJ, MacKerell AD (2000) Computation of the influence of chemical substitution on the pK a of pyridine using semiempirical and ab initio methods. Theo Chem Acc 103:483–494
Guven A (2005) Acidity study on 3-substituted pyridines. Int J Mol Sci 6:257–275
Blanco SE, Ferretti FH (2005) Calculation of acidity constants of pyridines in aqueous solution. Chem Phys Lett 403:400–404
Ögretir C, Tay NF, Özturk II (2007) A theoretical study on protonation of some halogen substituted pyridine derivatives. J Mol Graph Model 26:740–747
Rebollar-Zepeda AM, Galano A (2012) First principles calculations of pk a values of amines in aqueous solution: application to neurotransmitters. Int J Quantum Chem 112:3449–3460
Acknowledgments
This work was funded by the Spanish Government in the framework of Project CTQ2008-02207/BQU. One of us (R.C.) wishes to acknowledge a fellowship from the Spanish MEC within the FPU program. The authors are grateful to “Centro de Cálculo de Superomputación de Galicia” (CESGA) and to “Centre de Supercomputació de Catalunya” (CESCA) for access to their computational facilities.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published as part of the special collection of articles derived from the 8th Congress on Electronic Structure: Principles and Applications (ESPA 2012).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sastre, S., Casasnovas, R., Muñoz, F. et al. Isodesmic reaction for pK a calculations of common organic molecules. Theor Chem Acc 132, 1310 (2013). https://doi.org/10.1007/s00214-012-1310-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-012-1310-z