Abstract
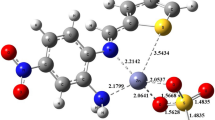
Chemically modified tetracyclines (CMTs) have shown promising activity as matrix metalloproteinase (MMP) inhibitors acting as zinc-binding groups. The first step in the design of new and effective drugs is the molecular description of the mechanism of action in chemical and biological environments. In the present study, the structure and stability of [Zn(LH n )(H2O)2]2−x (n = 0, 1, 2 and x = −2, −1, 0) and [Zn(L)(His)3], where L represents five distinct, structurally related CMTs, are discussed. In addition to the effect of the ligand on Zn(II) coordination, the role of the solvent and pH was also determined. The results suggested that O1–Oam (labeled as site II in the present paper) of CMT-1, CMT-4 and CMT-7 was the most stable site in the gas phase and aqueous solution. However, for CMT-3 and CMT-8, coordination at the O11–O12 moiety (site VI) was preferred. This coordination site is an essential binding mode of CMTs with active zinc in the MMP catalytic site; therefore, our results support the singular behavior of CMT-3 and CMT-8 as promising MMP inhibitors.





Similar content being viewed by others
References
Nuti E, Tuccinardi T, Rossello A (2007) Curr Pharm Des 13:2087–2100
Murphy G, Nagase H (2008) Mol Aspects Med 29:290–308
Roy R, Yang J, Moses MA (2009) J Clin Oncol 27:5287–5297
Tu GG, Xu WF, Huang HM, Li SH (2008) Curr Med Chem 15:1388–1395
Jacobsen JA, Jourden JLM, Miller MT, Cohen SM (2010) Biochim Biophys Acta 1803:72–94
Gupta SP (2007) Chem Rev 107:3042–3087
Rowsell S, Hawtin P, Minshull CA, Jepson H, Brockbank SMV, Barratt DG, Slater AM, McPheat WL, Waterson D, Henney AM, Pauptit RA (2002) J Mol Biol 319:173–181
Acharya MR, Venitz E, Figg WD, Sparreboom A (2004) Drug Resist. Updates 7:195–208
Syed S, Takimoto C, Hidalgo M, Rizzo J, Kuhn JG, Hammond LA, Schwartz G, Tolcher A, Patnaik A, Eckhardt SG, Rowinsky EK (2004) Clin Cancer Res 10:6512–6521
Lee H, Park JW, Kim SP, Lo EH, Lee SR (2009) Neurobiol Dis 34:189–198
Shen LC, Chen YK, Lin LM, Shaw SY (2010) Oral Oncol 46:178–184
Greenwald RA, Golub LM (2001) Curr Med Chem 8:237–242
Sandler C, Nurmi K, Lindstedt KA, Sorsa T, Golub LM, Kovanen PT, Eklund KK (2005) Int Immunopharmacol 5:1611–1621
Seftor REB, Seftor EA, De Larco JE, Kleiner DE, Leferson J, Stetler-Stevenson WG, McNamara TF, Golub LM, Hendrix MJC (1998) Clin Exp Metastasis 16:217–225
Liu Y, Ramamurthy NS, Marecek J, Lee HM, Chen JL, Ryan ME, Rifkin BR, Golub LM (2001) Curr Med Chem 8:243–252
Islam MM, Franco CD, Courtman DW, Bendeck MP (2003) Am J Pathol 163:1557–1566
Diaz N, Suarez D, Sordo TL (2006) J Phys Chem B 110:24222–24230
Aly AAM, Strasser A, Vogler A (2002) Inorg Chem Commun 5:411–413
Asleson GL, Frank CW (1975) J Am Chem Soc 97:6246–6248
De Siqueira JM, Carvalho S, Paniago EB, Tosi L, Beraldo H (1994) J Pharm Sci 83:291–295
Guerra W, Silva IR, Azevedo EA, Monteiro A, Bucciarelli-Rodriguez M, Chartone-Souza E, Silveira JN, Fontes APS, Pereira-Maia EC (2006) J Braz Chem Soc 17:1627–1633
Lee JY, Everett GW (1981) J Am Chem Soc 103:5221–5225
Machado FC, Demicheli C, Garniersuillerot A, Beraldo H (1995) J Inorg Biochem 60:163–173
Wessels JM, Ford WE, Szymczak W, Schneider S (1998) J Phys Chem B 102:9323–9331
Meindl K, Clark T (2005) J Phys Chem B 109:4279–4284
Leypold CF, Marian DT, Roman C, Schneider S, Schubert P, Scholz O, Hillen W, Clark T, Lanig H (2004) Photochem Photobiol Sci 3:109–119
Othersen OG, Lanig H, Clark T (2006) J Mol Model 12:953–963
Aleksandrov A, Simonson T (2006) J Comput Chem 27:1517–1533
Duarte HA, Carvalho S, Paniago EB, Simas AM (1999) J Pharm Sci 88:111–120
Marcial BL, Costa LAS, De Almeida WB, Dos Santos HF (2008) J Braz Chem Soc 19:1437–1449
Dos Santos HF, Marcial BL, De Miranda CF, Costa LAS, De Almeida WB (2006) J Inorg Biochem 100:1594–1605
Marcial BL, Costa LAS, De Almeida WB, Dos Santos HF (2009) J Mol Struct (Theochem) 916:94–104
Dos Santos HF, De Almeida WB, Zerner MC (1998) J Chem Soc Perkin Trans 2:2519–2525
Dos Santos HF, Xavier ES, Zerner MC, De Almeida WB (2000) J Mol Struct (Theochem) 527:193–202
De Almeida WB, Dos Santos HF, Zerner MC (1998) J Pharm Sci 87:1101–1108
Elkins PA, Ho YS, Smith WW, Janson CA, D’Alessio KJ, McQueney MS, Cummings MD, Romanic AM (2002) Acta Crystallogr D Biol Crystallogr 58:1182–1192
Pinsuwan S, Alvarez-Nunez FA, Tabibi SE, Yalkowsky SH (1999) J Pharm Sci 88:535–537
Becke AD (1988) Phys Rev A 38:3098–3100
Lee CT, Yang WT, Parr RG (1988) Phys Rev B 37:785–789
Curtiss LA, Raghavachari K, Redfern PC, Rassolov V, Pople JA (1998) J Chem Phys 109:7764–7776
Rassolov VA, Ratner MA, Pople JA, Redfern PC, Curtiss LA (2001) J Comput Chem 22:976–984
Sousa SF, Carvalho ES, Ferreira DM, Tavares IS, Fernandes PA, Ramos MJ, Gomes J (2009) J Comput Chem 30:2752–2763
Sousa SF, Fernandes PA, Ramos MJ (2007) J Phys Chem A 111:10439–10452
Zhao Y, Truhlar DG (2008) Theor Chem Acc 120:215–241
Cances E, Mennucci B, Tomasi J (1997) J Chem Phys 107:3032–3041
Cossi M, Scalmani G, Rega N, Barone V (2002) J Chem Phys 117:43–54
Barone V, Cossi M, Tomasi J (1997) J Chem Phys 107:3210–3221
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam NJ, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, revision A.1. Gaussian, Inc., Wallingford
Wiberg KB (1968) Tetrahedron 24:1083–1096
Tamames B, Sousa SF, Tamames J, Fernandes PA, Ramos MJ (2007) Proteins: Struct Funct Bioinform 69:466–475
Matos S, Beraldo H (1995) J Braz Chem Soc 6:405–411
Linder DP, Rodgers KR (2004) J Phys Chem B 108:13839–13849
Dos Santos HF, Nascimento CS, Belletato P, De Almeida WB (2003) J Mol Struct (Theochem) 626:305–319
Dos Santos HF, De Almeida WB, Zerner MC (1998) J Pharm Sci 87:190–195
Addison AW, Rao TN, Reedijk J, Vanrijn J, Verschoor GC (1984) J Chem Soc Dalton Trans 4:1349–1356
Sousa SF, Lopes AB, Fernandes PA, Ramos MJ (2009) Dalton Trans 48:7946–7956
Tochowicz A, Maskos K, Huber R, Oltenfreiter R, Dive V, Yiotakis A, Zanda M, Bode W, Goettig P (2007) J Mol Biol 371:989–1006
Acknowledgments
The authors thank the Conselho Nacional de Desenvolvimento Científico (CNPq—479682/2008-9) by the provision of the research concessions and for the financial support; and to Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG—CEX—APQ-00498-08) by the fomentation. B. L. Marcial also thanks to the CAPES for graduate fellowship.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
214_2010_881_MOESM1_ESM.doc
Optimized geometries of all complexes [Zn(LH n )(H2O)2]2−x (n = 0, 1, 2 and x = −2, −1, 0) are represented in Figs. S1–S2 for CMT-3 and Figs. S3–S4 for CMT-1. (DOC 2974 kb)
Rights and permissions
About this article
Cite this article
Marcial, B.L., Costa, L.A.S., De Almeida, W.B. et al. Interaction of chemically modified tetracyclines with catalytic Zn(II) ion in matrix metalloproteinase: evidence for metal coordination sites. Theor Chem Acc 128, 377–388 (2011). https://doi.org/10.1007/s00214-010-0881-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00214-010-0881-9