Abstract
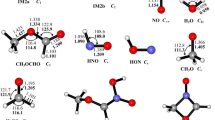
The radical-molecule reaction of C2Cl3 with NO2 is explored at the B3LYP/6-311G(d,p) and CCSD(T)/6-311+G(d,p) (single-point) levels. On the singlet potential energy surface (PES), the association between C2Cl3 and NO2 is found to be carbon-to-nitrogen attack forming the adduct C2Cl3NO2 (1) without any encounter barrier, followed by isomerization to C2Cl3ONO (2). Starting from 2, the most feasible pathway is the N–O1 bond cleavage which lead to P 1 (C2Cl3O + NO). Much less competitively, 2 transforms to the three-membered ring isomer c-OCCl2C–ClNO (4 a) which can easily interconvert to c-OCCl2C–ClNO 4 b. Then 4 (4 a, 4 b) takes direct C1–C2 and C2–O1 bonds cleavage to give P 2 (COCl2 + ClCNO). The lesser competitive channel is the 4 a isomerizes to the four-membered ring intermediate O-c-CNClOCCl2 (5) followed by dissociation to P3 (CO + ClNOCCl2). The concerted 1,2-Cl shift along with C1–O1 bond rupture of 4 b to form ONC(O)CCl3 (6) followed by dissociation to P 4 (ClNO + OCCCl2) is even much less feasible. Moreover, some of P 3 and P 4 can further dissociate to P 5 (ClNO + CO + CCl2). Compared with the singlet pathways, the triplet pathways may have less contribution to the title reaction. Our results are in marked difference from previous theoretical studies which showed that two initial adducts C2Cl3–NO2 and C2Cl3–ONO are obtained. Moreover, in the present paper we focus our main attentions on the cyclic isomers in view of only the chain-like isomers are considered by previous studies. The present study may be helpful for understanding the halogenated vinyl chemistry.






Similar content being viewed by others
References
Valeiras H, Gupta AK, Senkan SM (1984) Combust Sci Technol 36:123. doi:10.1080/00102208408923729
Senkan SM, Robinson JM, Gupta AK (1983) Combust Flame 49:305. doi:10.1016/0010-2180(83)90173-6
Chang WD, Senkan SM (1989) Environ Sci Technol 23:442. doi:10.1021/es00181a009
Taylor PH, Tirey DA, Dellinger B (1996) Combust Flame 104:260. doi:10.1016/0010-2180(95)00117-4
Taylor PH, Tirey DA, Rubey WA, Dellinger B (1994) Combust Sci Technol 101:75. doi:10.1080/00102209408951867
Russell JJ, Seetula JA, Gutman D, Senkan SM (1989) J Phys Chem 93:1935
Xiang TC, Liu KH, Zhao SL, Su HM, Kong FN, Wang BS (2007) J Phys Chem A 111:9606. doi:10.1021/jp074058c
Wang H, Li JC, Song XL, Li YZ, Hou H, Wang BS, Su HM, Kong FN (2006) J Phys Chem A 110:10336. doi:10.1021/jp0633345
Kostina KS, Bryukov MG, Shestov AA, Knyazev VD (2003) J Phys Chem A 107:1776. doi:10.1021/jp027162x
Xiang TC, Liu KH, Su HM (2007) Chin. J Chem Phys 20:407
Liu KH, Xiang TC, Wu WQ, Zhao SL, Su HM (2008) J Phys Chem A 112:10807. doi:10.1021/jp8031034
Mikhail GB, Sofya AK, Vadim DK (2003) J Phys Chem A 107:6574. doi:10.1021/jp034205g
Baren RE, Erickson MA, Hershberger JF (2002) Int J Chem Kinet 34:12. doi:10.1002/kin.10013
Rim KT, Hershberger JF (1998) J Phys Chem A 102:4592. doi:10.1021/jp981362k
Myerson AL (1975) In: 15th international symposium on combustion. The Combustion Institute, Pittsburgh, PA, p 1085
Song YH, Blair DW, Siminski VJ, Bartok W (1981) In: 18th international symposium on combustion. The Combustion Institute, Pittsburgh, PA, p 53
Chen SL, McCarthy JM, Clark WD, Heap MP, Seeker WR, Pershing DW (1986) In: 21st international symposium on combustion. The Combustion Institute, Pittsburgh, PA, p 1159
Zhang JX, Li ZS, Liu JY, Sun CC (2006) J Comput Chem 27:894. doi:10.1002/jcc.20397
Zhang JX, Li ZS, Liu JY, Sun CC (2007) Theory Chem Acc 117:579. doi:10.1007/s00214-006-0244-8
Eskola AJ, Geppert WD, Rissanen MP, Timonent RS, Halonen L (2005) J Phys Chem A 109:5376. doi:10.1021/jp050441a
Zhang JX, Liu JY, Li ZS, Sun CC (2005) J Comput Chem 26:807. doi:10.1002/jcc.20217
Yamada F, Slagle IR, Gutman D (1981) Chem Phys Lett 83:409. doi:10.1016/0009-2614(81)85490-5
Wollenhaupt M, Crowley JN (2000) J Phys Chem A 104:6429. doi:10.1021/jp0005726
Zhang JX, Li ZS, Liu JY, Sun CC (2006) J Comput Chem 27:661. doi:10.1002/jcc.20380
Seidler V, Temps F, Wagner HG, Wolf M (1989) J Phys Chem 93:1070. doi:10.1021/j100340a011
Darwin D, Moore CB (1995) J Phys Chem 99:13467. doi:10.1021/j100036a022
Liu JJ, Ding YH, Yu GT, Feng JK, Sun CC (2002) J Comput Chem 23:1031. doi:10.1002/jcc.10075
Wagal SS, Carrington T, Filseth SV, Sadowski CM (1982) Chem Phys 69:61. doi:10.1016/0301-0104(82)88132-9
Rim KT, Hershberger JF (1998) J Phys Chem A 102:4592. doi:10.1021/jp981362k
Yu GT, Ding YH, Li ZS, Huang XR, Sun CC (2001) J Phys Chem A 105:3388. doi:10.1021/jp003717h
Yu GT, Ding YH, Li ZS, Huang XR, Sun CC (2001) J Phys Chem A 105:9598. doi:10.1021/jp012481u
Van Hoeymissen J, De Boelpaep I, Uten W, Peeters J (1994) J Phys Chem 98:3725. doi:10.1021/j100065a030
Yu GT, Ding YH, Liu JY, Li ZS, Huang XR, Sun CC (2001) J Comput Chem 22:1907. doi:10.1002/jcc.1141
Cookson JL, Hancock G, Mckendrick KG, Bunsenges Ber (1985) Phys Chem 89:335. doi:10.1021/j100248a031
Zhang JX, Liu JY, Li ZS, Sun CC (2004) J Comput Chem 25:1888. doi:10.1002/jcc.20121
Baren RE, Erickson MA, Hershberger JF (2002) Int J Chem Kinet 34:12. doi:10.1002/kin.10013
Zhang JX, Liu JY, Li ZS, Sun CC (2004) J Comput Chem 25:1184. doi:10.1002/jcc.20043
Geppert WD, Eskola AJ, Timonen RS, Halonen L (2004) J Phys Chem A 108:4232. doi:10.1021/jp0370167
Du BN, Zhang WC (2006) J Mol Struct THEMCHEM 801:39
Frisch MJ, Trucks GW, Schlegel HB et al (1998) Gaussian 98, Revision A.6. Gaussian, Inc, Pittsburgh, PA
Acknowledgment
This work is supported by the National Natural Science Foundation of China (No. 20773048).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, Y., Liu, Hl., Huang, Xr. et al. Theoretical study on the mechanism of C2Cl3 + NO2 reaction. Theor Chem Acc 123, 431–441 (2009). https://doi.org/10.1007/s00214-009-0549-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00214-009-0549-5