Abstract
Cannabis self-administration studies may be helpful for identifying factors that influence cannabis consumption and subjective response to cannabis. Additionally, these paradigms could be useful for testing novel pharmacotherapies for cannabis use disorder. This scoping review aims to summarize the findings from existing ad libitum cannabis self-administration studies to determine what has been learned from these studies as well as their limitations. We examined studies that specifically examined cannabis smoking, focusing on subjective response and self-administration behavior (e.g., smoking topography). A systematic search was conducted using PubMed and Embase from inception to October 22, 2022. Our search strategy identified 26 studies (total N = 662, 79% male) that met our eligibility criteria. We found that tetrahydrocannabinol (THC) concentration significantly affected subjective response to cannabis in some but not all studies. In general, cannabis self-administration tended to be most intense at the beginning of the laboratory session and decreased in later parts of the session. There was limited data on cannabis self-administration in adults older than 55. Data on external validity and test-retest reliability were also limited. Addressing these limitations in future ad libitum cannabis self-administration studies could lead to more valid and generalizable paradigms, which in turn could be used to improve our understanding of cannabis use patterns and to help guide medication development for cannabis use disorder.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cannabis is one of the most commonly used drugs globally (Connor et al., 2021). In the past year, cannabis use among youth has increased in western nations, with prevalence rates of 18% (SAMHSA, 2021), 20% (European Monitoring Centre for Drugs and Drug Addiction, 2021), and 27% (Health Canada, 2021) in the USA, Europe, and Canada respectively. There is evidence that both rates of use and cannabis potency are rising in the USA. Between 2002 and 2020, past-year cannabis use in American adults aged 18 years and older has increased by about 8% (SAMHSA, 2021), and cannabis potency has increased since the 1980s from about 3% tetrahydrocannabinol (THC) to 12% THC in 2012 (Volkow et al., 2014). Along with this growing trend, the perception of risk from cannabis use has also decreased over the years, with fewer individuals associating harm with weekly cannabis use in 2020 (28.2%) compared to 2015 (38.7%) (SAMHSA, 2021). Long-term use of cannabis increases the risk of developing cannabis use disorder (CUD) (NIDA, 2021), and in the USA, the past-year prevalence of CUD has risen from 1.5% in 2001-2002 to 2.9% in 2012-2013 (Hasin et al., 2015).
Chronic cannabis use may increase the risk of other substance use disorders (Blanco et al., 2016) and increase the risk for and persistence of psychotic symptoms (Kuepper et al., 2011) and psychosocial impairment (Sorkhou et al., 2021). Given the adverse effects of cannabis, it is essential to gain a better understanding of how individuals self-administer the drug and its psychopharmacological effects. Although observational studies have been helpful in identifying the effects of cannabis, these studies have varying methodologies. They often rely on retrospective reports, which can be biased due to variations in the potency and type of cannabis used, co-use of other drugs, and recall bias. In contrast, cannabis self-administration (CSA) studies conducted in the laboratory allow for a potentially more valid examination of drug intake and subjective response, as researchers can control various external factors, such as cannabis potency and timing of administration. Understanding the reinforcing and subjective effects of cannabis may help us better understand who is most liable to develop CUD. Furthermore, reliable drug self-administration paradigms can be used for pharmacotherapy development (Panlilio et al., 2016; Ray et al., 2021). In order to model specific aspects of addiction, various drug self-administration designs have been developed, such as operant self-administration procedures in which participants are required to complete a task (e.g., pressing buttons) in order to receive the drugs (Haney, 2009; Stangl et al., 2022), controlled-smoking procedures (e.g., smoking inhalation guided by an experimenter’s instruction or cues) to standardize consumption (Kayser et al., 2021), choice procedures where participants are given the option to choose between the drug and one or more alternatives (e.g., money or other drugs) (Haney, 2009; Jones & Comer, 2013; McKee, 2009; Sloan et al., 2022), and free-access or ad libitum procedures (Gowin et al., 2017; Sloan et al., 2020). Ad libitum procedures are one of the most common self-administration paradigms (Chukwueke & Le Foll, 2019; Gowin et al., 2017; Jones & Comer, 2013; Sloan et al., 2020). In this type of study design, participants can freely administer a drug without restriction, although certain ceilings are often imposed for safety or practical reasons. In the present study, we specifically focused on ad libitum cannabis self-administration studies. Other forms of cannabis administration may also be reflective of real-world use (e.g., paradigms where participants pay for access to cannabis) but were beyond the scope of the current review.
The psychoactive effects of cannabis are derived from delta-9-tetrahydrocannabinol (THC). THC is rapidly absorbed into the bloodstream and peaks shortly after administration, usually in about 3-10 min when administered through inhalation (Grotenhermen, 2003). THC then acts as a partial agonist on cannabinoid receptor 1 (CB1) and cannabinoid receptor 2 (CB2) in the brain, with the psychoactive effects mediated by CB1 (Pacher et al., 2006; Sloan et al., 2019; Zou & Kumar, 2018). Cannabis can be administered recreationally through various methods, such as smoking, vaping, or oral ingestion. Although some studies have examined the effects of vaping (Spindle et al., 2019) and oral administration (Fogel et al., 2017), cannabis smoking remains the most common form of administration in both real-world (Health Canada, 2021) and laboratory settings (Russell et al., 2018; Vinette et al., 2022), so our review will focus on this administration method.
The present scoping review aims to summarize findings from ad libitum paradigms that have been used to study cannabis self-administration in the human laboratory to date. We will first summarize the design of ad libitum CSA studies and their subjective and behavioral findings. We will then discuss the test-retest reliability and external validity of these studies by reporting on correlations between repeated sessions and associations between cannabis use in the lab and external cannabis use. Finally, we will discuss the gaps in the literature and considerations for the design of future CSA studies.
Methods
Free-access or ad libitum paradigms are one of the most straightforward methods of measuring self-administration behavior. They are thought to be reflective of real-world use, as participants can consume the drug as desired. There are usually some restrictions such as a fixed timeframe or a maximum quantity of drug consumption allowed (i.e., ceiling) to ensure practicality and participant safety.
Included studies needed to be published in English and must have employed an ad libitum human laboratory cannabis self-administration paradigm with adult participants aged 18 years and older. In our review, we only included laboratory paradigms that allowed participants to smoke cannabis for at least 10 min. This minimum time window was selected to give sufficient time for participants to reach peak intoxication state (Grotenhermen, 2003). No CSA studies were found that were less than 10 min in duration. Studies must also have included information about either subjective response to cannabis or self-administration behavior (i.e., smoking topography or amount consumed). Studies that included co-administration of alcohol, other drugs, or other forms of cannabis (e.g., oral cannabis) were excluded as we aimed to specifically investigate studies probing the reinforcing effects of smoked cannabis in the absence of other drugs. Studies in which active drug or placebo were administered prior to cannabis self-administration were also excluded due to the possibility that the active drug or placebo would influence subjective response or self-administration behavior. CSA studies that looked at other forms of cannabis administration (e.g., oral administration, vaporization) or that only used other types of paradigms (e.g., controlled-smoking procedures) were also excluded. Studies in which the cannabis was self-supplied (e.g., mobile laboratory, local dispensaries) were excluded due to the lack of standardization in THC concentration. Studies with outcomes that were not related to either subjective response or CSA behavior, such as pharmacokinetic information or device sensitivity, were excluded.
The present scoping review was initially performed using PubMed from inception to March 7, 2021. An additional literature search was conducted on October 22, 2022 to include Embase. Relevant articles were determined from searching the title and abstract using the following keywords: “marijuana smoking,” “cannabis,” “self-administration,” “free-access,” and “ad libitum”. We also added the keywords “validity,” “reliability” and “reproducibility” after the final search strategy to see if we were able to capture any specific papers about external validity and test-retest reliability in ad libitum CSA studies. For additional information about the search strategy, see Supplementary Table 1.1 and 1.2. Additional articles were identified by checking citations in included papers. Articles were selected by two independent reviewers (initial search: KX and EG, second search: KX and AN). Abstracts were screened against the eligibility criteria using Covidence (Veritas Health Innovation, 2021), a web-based software. Duplicate papers were removed by Covidence. Discrepancies in study selection were resolved by a third author (MS). The data extraction was conducted by one author (KX) and verified by another author (EG for the initial extraction, AN and MS for the second extraction).
Our updated search identified 3727 papers, and based on title and abstract screening, 64 articles were selected. During the full-text screening, 34 papers were excluded; see details in Fig. 1 and Supplementary Table 2. For articles that consisted of only an abstract (e.g., conference papers) or that did not specify the type of self-administration used (e.g., ad libitum or controlled-smoking procedure), additional information was obtained by emailing the authors when possible.
Data extracted from the eligible papers included study design, sample size, mean age of the sample, the study’s inclusion criteria for baseline cannabis use, cannabis potency, requirements for abstinence prior to the CSA session, instructions to participants, and the duration of the ad libitum session. We also extracted outcome data related to subjective response and CSA behavior (i.e., smoking topography outcomes and amount of cannabis administered). Additional data were included if they were thought to be related to subjective response or CSA behavior. The study characteristics and results can be found in Table 1. Other Sources = articles from the preliminary search and in-text citations.
Results
Study selection
Thirty ad libitum CSA articles were found to be eligible (Fig. 1). Three studies had multiple articles (1 study had 3 papers (Brands et al. 2019; Matheson et al. 2020a, 2020b), and 2 studies had 2 papers each (Hoffman et al. 2021; Marcotte et al. 2022; Spindle et al. 2018, 2019)) resulting in a total of 26 ad libitum studies. Certain included studies were not fully ad libitum in that subjects were told to smoke a fixed amount (Herning et al. 1986; Heishman et al. 1989; Meyer et al. 1971; Miller et al. 1977a, 1977b, 1977c, 1977d, 1978, 1979; Miller and Cornett 1978; Perez-Reyes et al. 1981, 1982; Spindle et al. 2018, 2019, 2021; Schaefer et al. 1977). However, given that subjects were allowed to smoke the cannabis in any way that they wanted and that data derived from the studies looked at puff characteristics, other topography measures, and subjective response, it was felt that these articles contributed valuable information to our review, so the articles were included.
Study design
The included ad libitum studies were performed in either inpatient or outpatient units with various study designs (i.e., cross-over, sequential, single session, residential, and between-subject designs) and typically consisted of 5-20 participants. One group of investigators conducted studies with slightly larger sample sizes of 28-40 (Miller, Cornett, Brightwell, McFarland, Drew, et al., 1977; Miller, Cornett, Drew, McFarland, Brightwell, et al., 1977; Miller, Cornett, Brightwell, McFarland, Drew, et al., 1977; Miller, Cornett, Drew, McFarland, Brightwell, et al., 1977); the authors had noted that some of the participants were involved in more than one study (Miller & Cornett, 1978). Two studies had much larger sample sizes; one included 91 participants (Brands et al., 2019; Matheson, Mann, et al., 2020; Matheson, Sproule, et al., 2020) and another included 191 participants (Hoffman et al., 2021; Marcotte et al., 2022). In the cross-over and sequential studies, thirteen reported a separation period, usually ranging between 2 days to at least a week (Cappell et al., 1973; Chait, 1989; Heishman et al., 1989; Matthias et al., 1997; Meyer et al., 1971; Miller et al., 1978, 1979; Miller, McFarland, Cornett, Brightwell, et al., 1977; Miller & Cornett, 1978; Perez-Reyes et al., 1982; Spindle et al., 2018, 2019; Zacny & De Wit, 1991). Only one study had a separation period of 24 h (Miller, Cornett, Brightwell, McFarland, Drew, et al., 1977). To prevent residual (carryover) effects from any previous cannabis consumption, participants would often be asked to abstain from cannabis use anywhere from 6 h to 4 days before the ad libitum procedures (Brands et al., 2019; Cappell et al., 1973; Chait, 1989; Heishman et al., 1989; Hoffman et al., 2021; Marcotte et al., 2022; Matheson, Mann, et al., 2020; Matheson, Sproule, et al., 2020; Matthias et al., 1997; Miller et al., 1978; Miller, McFarland, Cornett, Brightwell, et al., 1977; Miller & Cornett, 1978; Wu et al., 1988), although this was not reported in all studies. The longest abstinence period was 11 days of detoxification prior to the study sessions (Tashkin et al., 1976). Abstinence was confirmed by clinical assessments (Herrmann et al., 2015), urine drug screen test (Brands et al., 2019; Matheson, Mann, et al., 2020; Matheson, Sproule, et al., 2020; Spindle et al., 2018, 2019), or oral fluid THC testing (Hoffman et al., 2021; Marcotte et al., 2022). Although most studies (17/26) provided participants with one cannabis cigarette for each ad libitum session, other studies offered multiple cigarettes per session, with cigarettes either provided at the start of the session or requested one at a time from the study staff. One paper had a residential study design with participants having the option to smoke as many cannabis cigarettes as desired for a total of 80 days (Tashkin et al., 1976). The length of the cigarette was not equivalent in all studies and only some studies measured the weight of each cigarette before and after use. Different studies used different instructions, which may also have affected participant behavior (Supplementary Table 3).
Participants
The study population typically consisted of heavy cannabis users between the ages of 18 and 55 (Table 1). The definition for heavy cannabis user varied across studies, with investigators defining heavy or frequent consumers as using cannabis at least twice per month (Schwope et al., 2012), at least two times per week (Herrmann et al., 2015), four or more times per week (Hoffman et al., 2021; Marcotte et al., 2022), or using cannabis almost daily (e.g., ≥ 25 days/month) (McClure et al., 2012; Meyer et al., 1971; Spindle et al., 2021). Reported baseline cannabis use often significantly exceeded minimum requirements as per inclusion criteria. Although the majority of our included studies did not address whether the participants were treatment-seeking, four studies (Brands et al., 2019; Chait, 1989; Hoffman et al., 2021; Marcotte et al., 2022; Matheson, Mann, et al., 2020; Matheson, Sproule, et al., 2020; Zacny & De Wit, 1991) did indicate that individuals with cannabis dependence or other substance use disorders would be excluded from participating in the study. Most studies (19/26) recruited entirely or predominantly (≥ 80%) male participants.
Subjective effects
Among the various types of subjective responses, drug “high” or “intoxication” were the most commonly measured. Subjective responses were often measured using a visual analog scale (VAS) or a simple 0-100 scale rating. Peak “high” usually occurred 5-30 min after exposure (Brands et al., 2019; Chait, 1989; Heishman et al., 1989; Herning et al., 1986; Matheson, Mann, et al., 2020; Matheson, Sproule, et al., 2020; Schwope et al., 2012; Spindle et al., 2018, 2019, 2021; Zacny & De Wit, 1991). The time for subjective effects to return to baseline differed across studies, from 4 h (Herrmann et al., 2015) to 6 h or more (Perez-Reyes et al., 1982; Schwope et al., 2012; Spindle et al., 2018, 2019).
Cannabis consumption
There are various methods for measuring the amount of cannabis consumed, such as the number of cigarettes administered, change in weight of cannabis cigarettes from pre-administration to post-administration, plasma THC levels, and smoking topography variables. Smoking topography examines how cannabis was smoked, such as smoking duration, puff number, and puff volume, which can be captured by observation or using a measurement tool like a single flow transducer, pneumotachograph, or spirometer. Twenty of our included studies assessed for smoking topography while 9 studies reported amount administered, and only 6 studies assessed for both (Brands et al., 2019; Cappell et al., 1973; Herrmann et al., 2015; Hoffman et al., 2021; Marcotte et al., 2022; Matheson, Mann, et al., 2020; Matheson, Sproule, et al., 2020; McClure et al., 2012; Perez-Reyes et al., 1982). In one study, when the participants were given higher potency cannabis (3.90% THC) (Herning et al., 1986), more puffs and larger inhalation volume were taken compared to lower potency cannabis (1.20% THC). In contrast, another study found that when participants smoked a higher potency (2.7% THC), the puff duration and volume were significantly reduced compared to the lower potency cannabis (1.3% THC) (Heishman et al., 1989). Six studies found no significant difference in smoking topography variables between different active THC potencies and between active and placebo cannabis (Cappell et al., 1973; Hoffman et al., 2021; Matthias et al., 1997; Perez-Reyes et al., 1982; Wu et al., 1988; Zacny & De Wit, 1991).
Studies consistently demonstrated that the longer one smokes, the lower the puff volume (Heishman et al., 1989; Herrmann et al., 2015; McClure et al., 2012; Wu et al., 1988). The start of the self-administration period may be the most intense, with puff volume and duration being higher in the first four puffs compared to the last four puffs of the cigarette (McClure et al., 2012). In another study, puff volume decreased 34% in the second half of the hour as compared to the first half (Herrmann et al., 2015). Similarly, when cannabis users were given 9 h of cannabis access, the puff volume and duration decreased with progressive puffs (McClure et al., 2012).
Cannabis consumption was usually measured by the number of cigarettes consumed or the weight of cigarettes before and after smoking. Only two studies reported data on cannabis craving (Herrmann et al., 2015; McClure et al., 2012), although one of these studies (McClure et al., 2012) only looked at craving during a forced abstinence period following cannabis self-administration rather than during the ad libitum session itself.
In terms of smoking duration, CSA sessions were at least 10 min in length. The longest single smoking session was 60 min (Herrmann et al., 2015). The longest ad libitum CSA period was a 94-day study on a closed research unit, where participants underwent 11 days of forced abstinence prior to 80 days of ad libitum use (except between day 76 and 82). In this study, participants consumed an average of 5.2 cannabis cigarettes daily (Tashkin et al., 1976).
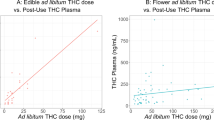
Multiple biological specimens can be used as objective indicators of cannabis use such as urine, blood, hair, and oral fluids. However, in human laboratory studies that require multiple samples at different time points, blood samples were most often used given the short detection window that could identify both the parent drug and their metabolites within minutes after exposure (Hadland & Levy, 2016). THC could be detected in the plasma within a minute after the first puff of a cannabis cigarette and peaked within 10 min (Musshoff & Madea, 2006). In our review, only seven studies measured blood THC levels (Brands et al., 2019; Herrmann et al., 2015; Hoffman et al., 2021; Marcotte et al., 2022; Matheson, Mann, et al., 2020; Matheson, Sproule, et al., 2020; Perez-Reyes et al., 1981, 1982; Schwope et al., 2012; Spindle et al., 2018, 2019). Three of these studies reported that peak subjective response (e.g., “high,” “stimulated,” “stoned,” “good drug effects,” “drug effects”) occurred when plasma THC levels peaked (Matheson, Sproule, et al., 2020; Schwope et al., 2012; Spindle et al., 2018, 2019). However, two studies with smaller sample size found that peak subjective response occurred after peak plasma THC levels were reached (Perez-Reyes et al., 1981, 1982).
Cannabis withdrawal
Cannabis withdrawal may begin within 1 day of abstinence (Budney et al., 2007; Connor et al., 2022), so some cannabis users may have already been in withdrawal at the start of the paradigm given that most ad libitum CSA studies required the participants to abstain from cannabis use before entering the laboratory. However, most studies did not measure withdrawal symptoms prior to self-administration to see if this impacted craving, subjective response, or self-administration behavior. Although one study (Spindle et al., 2021) did administer the Marijuana Withdrawal Checklist prior to the CSA session, the authors did not report whether it had any effect on subjective response or CSA behavior.
Test-retest reliability and external validity
There was limited data on the test-retest reliability and external validity of laboratory CSA. Although two studies (Chait, 1989; McClure et al., 2012) in our review had participants repeat the CSA sessions under the same conditions, neither study reported correlations in CSA behavior between the test sessions. One study (McClure et al., 2012) found that frequency of daily cannabis use in the past 30 days prior to the study was positively associated with total puff volume and maximum puff duration but no other smoking topography measures. Furthermore, the study found a correlation between years of cannabis use and total puff volume per cigarette, average volume per puff, and average puff duration. In a driving stimulation study (Hoffman et al., 2021; Marcotte et al., 2022), cannabis users with higher intensity of cannabis use in the past 6 months had the highest whole blood THC concentration post-smoking. This finding suggests that whole blood THC concentration during ad libitum consumption may be correlated with frequency of cannabis use outside of the laboratory.
Effects of cannabis potency
Compared to earlier studies, the potency of cannabis used in recent CSA studies has increased, with potencies as high as 13.4% being used (Hoffman et al., 2021; Marcotte et al., 2022; Spindle et al., 2018, 2019, 2021). Although higher potency cannabis resulted in significantly higher subjective responses (particularly “high” ratings) than their lower potency counterparts in most of our included studies (Cappell et al., 1973; Herning et al., 1986; Miller, Cornett, Drew, McFarland, Brightwell, et al., 1977; Miller & Cornett, 1978; Perez-Reyes et al., 1982; Schaefer et al., 1977; Zacny & De Wit, 1991), the largest study included in our review (N = 191) (Hoffman et al., 2021; Marcotte et al., 2022) found that individuals reported greater “high” in the 5.9% THC group than in the 13.4% THC group. The 5.9% THC group also achieved the highest blood THC concentration among the three potency groups (placebo, 5.9% THC, and 13.4% THC). Three papers (Chait, 1989; Heishman et al., 1989; Matthias et al., 1997) reported no significant difference in subjective response between the various cannabis potencies; however, this may have been due to the narrow concentration range (2.7% vs. 1.7% vs. 0.9% THC (Chait, 1989); 3.95% vs. 1.77% vs. 0.0% THC (Matthias et al., 1997); 2.7% vs. 1.3% (Heishman et al., 1989), making it difficult for participants to differentiate the various cannabis concentrations. These studies also had small sample sizes and were potentially underpowered to detect differences in subjective response between potencies.
Even though subjective response increases in a dose-dependent manner in some studies, there may still be no difference in smoking topography variables (Cappell et al., 1973; Matthias et al., 1997; Perez-Reyes et al., 1982; Zacny & De Wit, 1991) and amount consumed (Chait, 1989; Hoffman et al., 2021; Marcotte et al., 2022; Perez-Reyes et al., 1982). Only two studies in our review showed that cannabis potency affected smoking topography variables (e.g., puff and inhalation volume) (Heishman et al., 1989; Herning et al., 1986) and one study found an inverse relationship between cannabis potencies and amount smoked (Cappell et al., 1973). More research is required to determine cannabis potency’s effect on smoking topography variables and cannabis consumption.
Effects of sex
Included studies had a higher proportion of male participants. Only four studies examined sex differences between cannabis users (Matheson, Sproule, et al., 2020; Perez-Reyes et al., 1981, 1982; Spindle et al., 2019). Despite smoking for the same duration as male participants, female participants were found to smoke less of the cigarette, suggesting the female participants may be taking smaller and less frequent puffs than the male participants (Matheson, Sproule, et al., 2020). This supports an earlier small study (3 males, 3 females) which found that males took more puffs and consumed cigarettes more quickly than females, although it was not indicated whether these differences were statistically significant (Perez-Reyes et al., 1981). However, a later study found no difference in smoking pattern between the two sexes (Perez-Reyes et al., 1982). It has also been reported that females attain significantly lower maximum blood THC concentration than males (Matheson, Sproule, et al., 2020) and that females had numerically lower area under the plasma THC versus time curve than males (although the difference was not statistically significant) (Perez-Reyes et al., 1981). In contrast, two studies found that females achieved numerically higher blood THC concentrations than males (Perez-Reyes et al., 1982; Spindle et al., 2019). Although few differences in subjective response were identified between the sexes, “liking” and “feels like cannabis” ratings (Matheson, Sproule, et al., 2020) were higher in males. Despite having the same peak time and ratings, females’ ratings rapidly declined at 180 min and returned to baseline at 360 min (Matheson, Sproule, et al., 2020). Meanwhile, subjective response in males tended to persist longer, as they still had significantly different “liking” and “feels like cannabis” ratings from baseline at 360 min (Matheson, Sproule, et al., 2020).
Effects of environment
Few studies have examined the impact of surroundings and environment on CSA behavior. Some examples of potential influences could include the size and furnishings of the smoking room and the presence of other people (e.g., study staff or other cannabis smokers) in the room during self-administration. The influence of room ventilation on physiological, subjective, and behavioral effects of cannabis smoking was examined in one study (Herrmann et al., 2015). A group of cannabis smokers consumed a total of 2.1 g more cannabis in the ventilated condition (16.5 g total) than the unventilated condition (14.4 g total), but the authors did not address whether this was a statistically significant difference. There appeared to be no difference in subjective response (“feel drug effect”) by ventilation condition (Herrmann et al., 2015). Some studies had participants smoke in groups (Herrmann et al., 2015; Miller, Cornett, Brightwell, McFarland, Drew, et al., 1977; Miller, Cornett, Drew, McFarland, Brightwell, et al., 1977; Miller et al., 1979; Miller, McFarland, Cornett, & Brightwell, 1977), but it is unknown if this may have impacted smoking behavior.
Discussion
The objective of our scoping review was to provide an overview of ad libitum CSA studies as well as the limitations of these studies. From our included studies, we found there was a high level of heterogeneity in ad libitum study designs, with differences in self-administration instructions, length of administration, and smoking environment. These factors could influence CSA outcomes; however, it is difficult to determine how important these factors are given the lack of comparative research. Outcome variables also differed between studies. For example, some ad libitum studies measure smoking topography variables such as puff number, puff volume, and inhalation duration, while others only measure the number of cigarettes smoked or the weight of the cigarette before and after smoking. The differences in design and measurement make it challenging to compare across studies.
The lack of major differences in smoking topography outcomes between different cannabis potencies (Hoffman et al., 2021; Matthias et al., 1997; Wu et al., 1988; Zacny & De Wit, 1991) is surprising. The few studies that found differences between cannabis potencies (Heishman et al., 1989; Herning et al., 1986) suggest that some smoking titration may be involved. The study with the largest sample size reported that individuals experienced a greater “high” in the 5.9% THC group compared to the 13.4% THC group (Hoffman et al., 2021; Marcotte et al., 2022), suggesting that potency may not be the only factor that determines “high,” but other factors (e.g., inhalation volume) might play an important role (especially since individuals in the 5.9% THC group had higher blood THC concentrations than those in the 13.4% THC group in this study).
None of the papers we reviewed examined the effects of age on subjective response, cannabis consumption, or smoking topography. Most study samples consisted of adults less than 40 years of age, with few studies examining CSA in older adults. The use of cannabis in adults age 65 and older has been gradually increasing from 0.4% in 2006 (Han et al., 2017) to about 2.9% by 2016 (Han & Palamar, 2018). A recent study (Mueller et al., 2021) with recreational users reported that THC-dominant cannabis use in older adults (age 55-70) had less detrimental effects on learning and processing speed tests than in younger adults (age 21-25). However, older adults may be more likely to develop sedation, reduced consciousness, lightheadedness, and weakness/inability to stand after acute cannabis exposure compared to younger adults (age 19-59) (Hendrickson et al., 2020), which may require more attention and care during CSA. Cannabis use has also been linked to higher odds of myocardial infarction, coronary artery disease, and stroke in older adults (Shah et al., 2021) who are already more vulnerable to cardiovascular events (Latif & Garg, 2020; Rodgers et al., 2019). This may be a barrier to studying cannabis self-administration in older adults, especially in individuals at heightened risk of potential cardiovascular sequelae, such as those with pre-existing cardiovascular disease.
Subjects in ad libitum paradigms were also predominately male, with the percentage of male participants usually ranging between 70 and 100%. Given varying CSA behaviors in females and males in preclinical research (Fattore et al., 2007), it is important that females be represented in this literature. Only four ad libitum studies examined the effects of sex on subjective response and cannabis consumption (Matheson, Sproule, et al., 2020; Perez-Reyes et al., 1981, 1982; Spindle et al., 2019). In these studies, findings on smoking behavior, subjective response, and pharmacokinetic profiles between the sexes were contradictory (Matheson, Sproule, et al., 2020; Perez-Reyes et al., 1981, 1982; Spindle et al., 2019). Clearly, more research comparing CSA behavior in males and females is needed.
Other factors that may impact CSA outcomes are withdrawal, acute craving, tolerance, sleep quality, stress, anxiety, and mood. These factors have been understudied in ad libitum CSA paradigms to date. One study found a negative correlation between puff duration and sleep quality (McClure et al., 2012). Only one study assessed cannabis withdrawal, but this study did not report whether there were any associations with subjective response or consumption (Spindle et al., 2021).
Perhaps most importantly, our review reveals limited assessment of test-retest reliability and external validity for most ad libitum CSA paradigms. From our included ad libitum studies, only two studies repeated CSA paradigms at least twice under the same condition on different days (Chait, 1989; McClure et al., 2012); however, neither study reported whether consumption behavior or subjective response were correlated between sessions. Only two studies (Hoffman et al., 2021; Marcotte et al., 2022; McClure et al., 2012) examined the validity of laboratory cannabis consumption by comparing the puff volumes or blood THC measures during the ad libitum period with external consumption. Insufficient verification of external validity raises the possibility that laboratory results might be an inaccurate representation of real-world CSA behavior; therefore, more data on the reliability and validity of these paradigms is critical to determine their real-world usefulness. Using validated self-report assessments (e.g., Timeline Followback, Marijuana Craving Questionnaire) or real-time report (e.g., Ecological Momentary Assessment (Trull et al., 2022)) prior to the CSA session and comparing these measures to related outcomes in the laboratory could help confirm external validity. Test-retest reliability could be measured using within-subject designs where participants repeat the same CSA session at two or more time points to see if self-administration behavior, peak THC levels achieved, craving, and subjective response are correlated between the sessions and the magnitude of these correlations.
Our review is limited by the fact that it focused primarily on ad libitum paradigms that investigated subjective response and self-administration behavior. Reviews focused on other outcomes such as medication effects or driving are needed to determine whether CSA paradigms are reliable and externally valid for those specific outcomes. Some cannabis-related outcomes might be better assessed by employing other types of drug self-administration paradigms such as controlled-smoking procedures or choice procedures where individuals pay for access to cannabis, but these were not reviewed here.
Based on the results of our review, we found that there is a high level of heterogeneity across ad libitum CSA studies. Self-administration behavior in ad libitum studies appeared to be most intense in the early part of laboratory sessions and decreased in the latter parts of the session, suggesting that users may reach their desired high early on during self-administration. Data on test-retest reliability and external validity were limited. Test-retest reliability and external validity data should be collected when developing and evaluating novel paradigms to ensure that they reliably reflect real-world CSA behavior. CSA studies in older adult and female samples are needed to better understand cannabis administration in these demographic groups. Future ad libitum CSA studies should also collect data on craving and cannabis withdrawal to determine the impact of these measures on self-administration. More thoughtful design of ad libitum CSA studies could lead to better quality data and improved paradigms, which may help us understand why certain individuals are at risk for developing CUD and lead to effective platforms to test novel pharmacotherapies and interventions for cannabis use disorder.
References
Blanco C, Hasin DS, Wall MM, Flórez-Salamanca L, Hoertel N, Wang S, Kerridge BT, Olfson M (2016) Cannabis use and risk of psychiatric disorders: prospective evidence from a US national longitudinal study. JAMA Psychiatr 73(4):388–395. https://doi.org/10.1001/jamapsychiatry.2015.3229
Brands B, Mann RE, Wickens CM, Sproule B, Stoduto G, Sayer GS, Burston J, Pan JF, Matheson J, Stefan C, George TP, Huestis MA, Rehm J, Le Foll B (2019) Acute and residual effects of smoked cannabis: impact on driving speed and lateral control, heart rate, and self-reported drug effects. Drug Alcohol Depend 205:107641. https://doi.org/10.1016/j.drugalcdep.2019.107641
Budney AJ, Roffman R, Stephens RS, Walker D (2007) Marijuana dependence and its treatment. Addict Sci Clin Pract 4(1):4–16. https://doi.org/10.1151/ascp07414
Cappell H, Kuchar E, Webster CD (1973) Some correlates of marihuana self-administration in man: a study of titration of intake as a function of drug potency. Psychopharmacologia 29(3):177–184. https://doi.org/10.1007/BF00414031
Chait LD (1989) Delta-9-tetrahydrocannabinol content and human marijuana self-administration. Psychopharmacology 98(1):51–55. https://doi.org/10.1007/BF00442005
Chukwueke CC, Le Foll B (2019) The human laboratory and drug development in alcohol use disorder: recent updates. Methods in Molecular Biology (Clifton, NJ) 2011:195–219. https://doi.org/10.1007/978-1-4939-9554-7_12
Connor JP, Stjepanović D, Le Foll B, Hoch E, Budney AJ, Hall WD (2021) Cannabis use and cannabis use disorder. Nature Reviews Disease Primers 7(1):16. https://doi.org/10.1038/s41572-021-00247-4
Connor JP, Stjepanović D, Budney AJ, Le Foll B, Hall WD (2022) Clinical management of cannabis withdrawal. Addiction 117(7):2075–2095. https://doi.org/10.1111/add.15743
European Monitoring Centre for Drugs and Drug Addiction (2021) Cannabis: health and social responses. In Health and social responses to drug problems: A European guide 2021. https://www.emcdda.europa.eu/publications/mini-guides/cannabis-health-and-social-responses_en#section3
Fattore L, Spano MS, Altea S, Angius F, Fadda P, Fratta W (2007) Cannabinoid self-administration in rats: sex differences and the influence of ovarian function. Br J Pharmacol 152(5):795–804. https://doi.org/10.1038/sj.bjp.0707465
Fogel JS, Kelly TH, Westgate PM, Lile JA (2017) Sex differences in the subjective effects of oral Δ9-THC in cannabis users. Pharmacol Biochem Behav 152:44–51. https://doi.org/10.1016/j.pbb.2016.01.007
Gowin JL, Sloan ME, Stangl BL, Vatsalya V, Ramchandani VA (2017) Vulnerability for alcohol use disorder and rate of alcohol consumption. Am J Psychiatr 174(11):1094–1101. https://doi.org/10.1176/appi.ajp.2017.16101180
Grotenhermen F (2003) Pharmacokinetics and pharmacodynamics of cannabinoids. Clin Pharmacokinet 42(4):327–360. https://doi.org/10.2165/00003088-200342040-00003
Hadland SE, Levy S (2016) Objective testing: urine and other drug tests. Child Adolesc Psychiatr Clin N Am 25(3):549–565. https://doi.org/10.1016/j.chc.2016.02.005
Han BH, Palamar JJ (2018) Marijuana use by middle-aged and older adults in the United States, 2015-2016. Drug Alcohol Depend 191:374–381. https://doi.org/10.1016/j.drugalcdep.2018.07.006
Han BH, Sherman S, Mauro PM, Martins SS, Rotenberg J, Palamar JJ (2017) Demographic trends among older cannabis users in the United States, 2006-13. Addiction 112(3):516–525. https://doi.org/10.1111/add.13670
Haney M (2009) Self-administration of cocaine, cannabis and heroin in the human laboratory: benefits and pitfalls. Addict Biol 14(1):9–21. https://doi.org/10.1111/j.1369-1600.2008.00121.x
Hasin DS, Saha TD, Kerridge BT, Goldstein RB, Chou SP, Zhang H, Jung J, Pickering RP, Ruan WJ, Smith SM, Huang B, Grant BF (2015) Prevalence of marijuana use disorders in the United States between 2001-2002 and 2012-2013. JAMA Psychiatry 72(12):1235–1242. https://doi.org/10.1001/jamapsychiatry.2015.1858
Health Canada (2021) Canadian Cannabis Survey 2020: Summary. https://www.canada.ca/en/health-canada/services/drugs-medication/cannabis/research-data/canadian-cannabis-survey-2020-summary.html
Heishman SJ, Stitzer ML, Yingling JE (1989) Effects of tetrahydrocannabinol content on marijuana smoking behavior, subjective reports, and performance. Pharmacol Biochem Behav 34(1):173–179. https://doi.org/10.1016/0091-3057(89)90369-9
Herning RI, Hooker WD, Jones RT (1986) Tetrahydrocannabinol content and differences in marijuana smoking behavior. Psychopharmacology 90(2). https://doi.org/10.1007/BF00181232
Hendrickson RG, McKeown NJ, Kusin SG, Lopez AM (2020) Acute cannabis toxicity in older adults. Toxicol Commun 4(1):67–70. https://doi.org/10.1080/24734306.2020.1852821
Herrmann ES, Cone EJ, Mitchell JM, Bigelow GE, LoDico C, Flegel R, Vandrey R (2015) Non-smoker exposure to secondhand cannabis smoke II: effect of room ventilation on the physiological, subjective, and behavioral/cognitive effects. Drug Alcohol Depend 151:194–202. https://doi.org/10.1016/j.drugalcdep.2015.03.019
Hoffman MA, Hubbard JA, Sobolesky PM, Smith BE, Suhandynata RT, Sanford S, Sones EG, Ellis S, Umlauf A, Huestis MA, Grelotti DJ, Grant I, Marcotte TD, Fitzgerald RL (2021) Blood and oral fluid cannabinoid profiles of frequent and occasional cannabis smokers. J Anal Toxicol 45(8):851–862. https://doi.org/10.1093/jat/bkab078
Jones JD, Comer SD (2013) A review of human drug self-administration procedures. Behav Pharmacol 24(5–6):384–395. https://doi.org/10.1097/FBP.0b013e3283641c3d
Kayser RR, Haney M, Simpson HB (2021) Human laboratory models of cannabis use: applications for clinical and translational psychiatry research. Front Psychiatry 12:626150. https://doi.org/10.3389/fpsyt.2021.626150
Kuepper R, van Os J, Lieb R, Wittchen H-U, Hofler M, Henquet C (2011) Continued cannabis use and risk of incidence and persistence of psychotic symptoms: 10 year follow-up cohort study. BMJ 342:d738–d738. https://doi.org/10.1136/bmj.d738
Latif Z, Garg N (2020) The impact of marijuana on the cardiovascular system: a review of the most common cardiovascular events associated with marijuana use. J Clin Med 9(6):1925. https://doi.org/10.3390/jcm9061925
Marcotte TD, Umlauf A, Grelotti DJ, Sones EG, Sobolesky PM, Smith BE, Hoffman MA, Hubbard JA, Severson J, Huestis MA, Grant I, Fitzgerald RL (2022) Driving performance and cannabis users’ perception of safety: a randomized clinical trial. JAMA Psychiatry 79(3):201. https://doi.org/10.1001/jamapsychiatry.2021.4037
Matheson J, Mann RE, Sproule B, Huestis MA, Wickens CM, Stoduto G, George TP, Rehm J, Le Foll B, Brands B (2020a) Acute and residual mood and cognitive performance of young adults following smoked cannabis. Pharmacol Biochem Behav 194:172937. https://doi.org/10.1016/j.pbb.2020.172937
Matheson J, Sproule B, Di Ciano P, Fares A, Le Foll B, Mann RE, Brands B (2020b) Sex differences in the acute effects of smoked cannabis: evidence from a human laboratory study of young adults. Psychopharmacology 237(2):305–316. https://doi.org/10.1007/s00213-019-05369-y
Matthias P, Tashkin DP, Marques-Magallanes JA, Wilkins JN, Simmons MS (1997) Effects of varying marijuana potency on deposition of tar and ⌬9-THC in the lung during smoking. 6
McClure EA, Stitzer ML, Vandrey R (2012) Characterizing smoking topography of cannabis in heavy users. Psychopharmacology 220(2):309–318. https://doi.org/10.1007/s00213-011-2480-4
McKee SA (2009) Developing human laboratory models of smoking lapse behavior for medication screening. Addict Biol 14(1):99–107. https://doi.org/10.1111/j.1369-1600.2008.00135.x
Meyer RE, Pillard RC, Shapiro LM, Mirin SM (1971) Administration of marijuana to heavy and casual marijuana users. Am J Psychiatr 128(2):198–204. https://doi.org/10.1176/ajp.128.2.198
Miller LL, Cornett TL (1978) Marijuana: dose effects on pulse rate, subjective estimates of intoxication, free recall and recognition memory. Pharmacol Biochem Behav 9(5):573–577. https://doi.org/10.1016/0091-3057(78)90205-8
Miller LL, Cornett TL, Brightwell DR, McFarland DJ, Drew WD, Wikler A (1977a) Marijuana: effects on storage and retrieval of prose material. Psychopharmacology 51(3):311–316. https://doi.org/10.1007/BF00431642
Miller LL, Cornett TL, Drew WD, McFarland DJ, Brightwell DR, Wikler A (1977b) Marijuana: dose-response effects on pulse rate, subjective estimates of potency, pleasantness, and recognition memory. Pharmacology 15(3):268–275. https://doi.org/10.1159/000136698
Miller LL, McFarland DJ, Cornett TL, Brightwell DR (1977c) Marijuana and memory impairment: effect on free recall and recognition memory. Pharmacol Biochem Behav 7(2):99–103. https://doi.org/10.1016/0091-3057(77)90191-5
Miller LL, McFarland DJ, Cornett TL, Brightwell DR, Wikler A (1977d) Marijuana: effects on free recall and subjective organization of pictures and words. Psychopharmacology 55(3):257–262. https://doi.org/10.1007/BF00497857
Miller LL, Cornett TL, McFarland DJ (1978) Marijuana: an analysis of storage and retrieval deficits in memory with the technique of restricted reminding. Pharmacol Biochem Behav 8(4):327–332. https://doi.org/10.1016/0091-3057(78)90065-5
Miller LL, Cornett TL, Wikler A (1979) Marijuana: effects on pulse rate, subjective estimates of intoxication and multiple measures of memory. Life Sci 25(15):1325–1330. https://doi.org/10.1016/0024-3205(79)90398-9
Mirin SM, Shapiro LM, Meyer RE, Pillard RC, Fisher S (1971) Casual versus heavy use of marijuana: a redefinition of the marijuana problem. Am J Psychiatry 127(9):1134–1140. https://doi.org/10.1176/ajp.127.9.1134
Mueller RL, Ellingson JM, Bidwell LC, Bryan AD, Hutchison KE (2021) Are the acute effects of THC different in aging adults? Brain Sciences 11(5):590. https://doi.org/10.3390/brainsci11050590
Musshoff F, Madea B (2006) Review of biologic matrices (urine, blood, hair) as indicators of recent or ongoing cannabis use. Ther Drug Monit 28(2):155–163. https://doi.org/10.1097/01.ftd.0000197091.07807.22
NIDA (2021) Is there a link between marijuana use and psychiatric disorders? https://www.drugabuse.gov/publications/research-reports/marijuana/there-link-between-marijuana-use-psychiatric-disorders
Pacher P, Bátkai S, Kunos G (2006) The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev 58(3):389–462. https://doi.org/10.1124/pr.58.3.2
Panlilio LV, Justinova Z, Trigo JM, Le Foll B (2016) Screening medications for the treatment of cannabis use disorder. Int Rev Neurobiol 126:87–120. https://doi.org/10.1016/bs.irn.2016.02.005
Perez-Reyes M, Owens SM, Di Guiseppi S (1981) The clinical pharmacology and dynamics of marihuana cigarette smoking. J Clin Pharmacol 21(S1):201S–207S. https://doi.org/10.1002/j.1552-4604.1981.tb02596.x
Perez-Reyes M, Guiseppi SD, Davis KH, Schindler VH, Edgar Cook C (1982) Comparison of effects of marihuana cigarettes of three different potencies. Clin Pharmacol Ther 31(5):617–624. https://doi.org/10.1038/clpt.1982.86
Ray LA, Du H, Green R, Roche DJO, Bujarski S (2021) Do behavioral pharmacology findings predict clinical trial outcomes? A proof-of-concept in medication development for alcohol use disorder. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology 46(3):519–527. https://doi.org/10.1038/s41386-020-00913-3
Rodgers JL, Jones J, Bolleddu SI, Vanthenapalli S, Rodgers LE, Shah K, Karia K, Panguluri SK (2019) Cardiovascular risks associated with gender and aging. J Cardiovasc Dev Dis 6(2):19. https://doi.org/10.3390/jcdd6020019
Russell C, Rueda S, Room R, Tyndall M, Fischer B (2018) Routes of administration for cannabis use – basic prevalence and related health outcomes: a scoping review and synthesis. Int J Drug Policy 52:87–96. https://doi.org/10.1016/j.drugpo.2017.11.008
SAMHSA. (2021). 2020 National Survey of Drug Use and Health (NSDUH): detailed tables.
Schaefer CF, Gunn CG, Dubowski KM (1977) Dose-related heart-rate, perceptual, and decisional changes in man following marihuana smoking. Percept Mot Skills 44(1):3–16. https://doi.org/10.2466/pms.1977.44.1.3
Schwope DM, Bosker WM, Ramaekers JG, Gorelick DA, Huestis MA (2012) Psychomotor performance, subjective and physiological effects and whole blood 9-tetrahydrocannabinol concentrations in heavy, chronic cannabis smokers following acute smoked cannabis. J Anal Toxicol 36(6):405–412. https://doi.org/10.1093/jat/bks044
Shah S, Patel S, Paulraj S, Chaudhuri D (2021) Association of marijuana use and cardiovascular disease: a behavioral risk factor surveillance system data analysis of 133,706 US adults. Am J Med 134(5):614–620.e1. https://doi.org/10.1016/j.amjmed.2020.10.019
Sloan ME, Grant CW, Gowin JL, Ramchandani VA, Le Foll B (2019) Endocannabinoid signaling in psychiatric disorders: a review of positron emission tomography studies. Acta Pharmacol Sin 40(3):342–350. https://doi.org/10.1038/s41401-018-0081-z
Sloan ME, Gowin JL, Janakiraman R, Ester CD, Stoddard J, Stangl B, Ramchandani VA (2020) High-risk social drinkers and heavy drinkers display similar rates of alcohol consumption. Addict Biol 25(2):e12734. https://doi.org/10.1111/adb.12734
Sloan ME, Sells JR, Vaughan CL, Morris JK, Ortega NE, Sundar S, Soundararajan S, Stangl BL, Gowin J, Chawla S, Diazgranados N, McKee SA, Waters A, Ramchandani VA (2022) Modeling ability to resist alcohol in the human laboratory: a pilot study. Drug Alcohol Depend Rep 5:100105. https://doi.org/10.1016/j.dadr.2022.100105
Sorkhou M, Bedder RH, George TP (2021) The behavioral sequelae of cannabis use in healthy people: a systematic review. Front Psychiatry 12:630247. https://doi.org/10.3389/fpsyt.2021.630247
Spindle TR, Cone EJ, Schlienz NJ, Mitchell JM, Bigelow GE, Flegel R, Hayes E, Vandrey R (2018) Acute effects of smoked and vaporized cannabis in healthy adults who infrequently use cannabis: a crossover trial. JAMA Netw Open 1(7):e184841. https://doi.org/10.1001/jamanetworkopen.2018.4841
Spindle TR, Cone EJ, Schlienz NJ, Mitchell JM, Bigelow GE, Flegel R, Hayes E, Vandrey R (2019) Acute pharmacokinetic profile of smoked and vaporized cannabis in human blood and oral fluid. J Anal Toxicol 43(4):233–258. https://doi.org/10.1093/jat/bky104
Spindle TR, Kuwabara H, Eversole A, Nandi A, Vandrey R, Antoine DG, Umbricht A, Guarda AS, Wong DF, Weerts EM (2021) Brain imaging of cannabinoid type I (CB 1 ) receptors in women with cannabis use disorder and male and female healthy controls. Addict Biol 26(6). https://doi.org/10.1111/adb.13061
Stangl BL, Byrd ND, Soundararajan S, Plawecki MH, O’Connor S, Ramchandani VA (2022) The motivation for alcohol reward: predictors of progressive-ratio intravenous alcohol self-administration in humans. J Visualized Experiments: JoVE 182. https://doi.org/10.3791/63576
Tashkin DP, Shapiro BJ, Lee YE, Harper CE (1976) Subacute effects of heavy marihuana smoking on pulmonary function in healthy men. N Engl J Med 294(3):125–129. https://doi.org/10.1056/NEJM197601152940302
Trull TJ, Freeman LK, Fleming MN, Vebares TJ, Wycoff AM (2022) Using ecological momentary assessment and a portable device to quantify standard tetrahydrocannabinol units for cannabis flower smoking. Addiction 117(8):2351–2358. https://doi.org/10.1111/add.15872
Vandrey R, Smith MT, McCann UD, Budney AJ, Curran EM (2011) Sleep disturbance and the effects of extended-release zolpidem during cannabis withdrawal. Drug Alcohol Depend 117(1):38–44. https://doi.org/10.1016/j.drugalcdep.2011.01.003
Veritas Health Innovation (2021) Covidence better systematic review management. https://www.covidence.org
Vinette B, Côté J, El-Akhras A, Mrad H, Chicoine G, Bilodeau K (2022) Routes of administration, reasons for use, and approved indications of medical cannabis in oncology: a scoping review. BMC Cancer 22(1):319. https://doi.org/10.1186/s12885-022-09378-7
Volkow ND, Baler RD, Compton WM, Weiss SRB (2014) Adverse health effects of marijuana use. N Engl J Med 370(23):2219–2227. https://doi.org/10.1056/NEJMra1402309
Wu T-C, Tashkin DP, Rose JE, Djahed B (1988) Influence of marijuana potency and amount of cigarette consumed on marijuana smoking pattern. J Psychoactive Drugs 20(1):43–46. https://doi.org/10.1080/02791072.1988.10524370
Zacny JP, De Wit H (1991) Effects of food deprivation on subjective effects and self-administration of marijuana in humans. Psychol Rep 68:12
Zou S, Kumar U (2018) Cannabinoid receptors and the endocannabinoid system: signaling and function in the central nervous system. Int J Mol Sci 19(3):E833. https://doi.org/10.3390/ijms19030833
Acknowledgements
We would like to thank all the authors we contacted who took the time to provide us with any clarifications and missing information that was necessary for our review.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Dr. Bernard Le Foll has obtained funding from Pfizer Inc. (GRAND Awards, including salary support) for investigator-initiated projects. Dr Le Foll has obtained funding from Indivior for a clinical trial sponsored by Indivior. Dr. Le Foll has in-kind donations of cannabis products from Aurora Cannabis Enterprises Inc. and study medication donations from Pfizer Inc. (varenicline for smoking cessation) and Bioprojet Pharma. He was also provided a coil for a transcranial magnetic stimulation (TMS) study from Brainsway. Dr. Le Foll has obtained industry funding from Canopy Growth Corporation (through research grants handled by the Centre for Addiction and Mental Health and the University of Toronto), Bioprojet Pharma, Alcohol Countermeasure Systems (ACS), Alkermes, and Universal Ibogaine. Lastly, Dr. Le Foll has received in-kind donations of nabiximols from GW Pharmaceuticals for past studies funded by CIHR and NIH.
Dr. Le Foll has participated in a session of a National Advisory Board Meeting (Emerging Trends BUP-XR) for Indivior Canada and has been a consultant for Shinogi. He is supported by CAMH, Waypoint Centre for Mental Health Care, a clinician-scientist award from the Department of Family and Community Medicine of the University of Toronto and a Chair in Addiction Psychiatry from the Department of Psychiatry of the University of Toronto.
Dr. Tony George is the chair of the scientific advisory committee of the Canadian Centre for Substance Use and Addiction (CCSA) and is compensated for cannabis-related policy work. Dr. George is also the co-principal editor of the journal Neuropsychopharmacology.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1:
Supplementary Table 1.1. PubMed Search Strategy Conducted on October 22, 2022. Supplementary Table 1.2. Embase Search Strategy Conducted on October 22, 2022. Supplementary Table 1.3. Initial PUBMED Search Strategy Conducted on March 7, 2021. Supplementary Table 2. Excluded CSA Ad libitum articles (n= 34). Supplementary Table 3. Instructions Used for Ad Libitum smoking (DOCX 41 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xiao, K.B., Grennell, E., Ngoy, A. et al. Cannabis self-administration in the human laboratory: a scoping review of ad libitum studies. Psychopharmacology 240, 1393–1415 (2023). https://doi.org/10.1007/s00213-023-06360-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-023-06360-4