Abstract
Rationale
Gamma-aminobutyric acid (GABA)ergic neurons of the substantia nigra pars reticulata (SNpr) are connected to the deep layers of the superior colliculus (dlSC). The dlSC, in turn, connect with the SNpr through opioid projections. Nociceptin/orphanin FQ peptide (N/OFQ) is a natural ligand of a Gi protein-coupled nociceptin receptor (ORL1; NOP) that is also found in the SNpr. Our hypothesis is that tectonigral opioid pathways and intranigral orphanin-mediated mechanisms modulate GABAergic nigrotectal connections.
Objectives
Therefore, the aim of this work was to study the role of opioid and NOP receptors in the SNpr during the modulation of defence reactions organised by the dlSC.
Methods
The SNpr was pretreated with either opioid or NOP receptor agonists and antagonists, followed by dlSC treatment with bicuculline.
Results
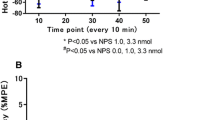
Blockade of GABAA receptors in the dlSC elicited fear-related defensive behaviour. Pretreatment of the SNpr with naloxone benzoylhydrazone (NalBzoH), a μ-, δ-, and κ1-opioid receptor antagonist as well as a NOP receptor antagonist, decreased the aversive effect of bicuculline treatment on the dlSC. Either μ-opioid receptor activation or blockade by SNpr microinjection of endomorphin-1 (EM-1) and CTOP promoted pro-aversive and anti-aversive actions, respectively, that modulated the defensive responses elicited by bicuculline injection into the dlSC. Pretreatment of the SNpr with the selective NOP receptor antagonist JTC801 decreased the aversive effect of bicuculline, and microinjections of the selective NOP receptor agonist NNC 63-0532 promoted the opposite effect.
Conclusions
These results demonstrate that opioid pathways and orphanin-mediated mechanisms have a critical role in modulating the activity of nigrotectal GABAergic pathways during the organisation of defensive behaviours.






Similar content being viewed by others
Abbreviations
- dlSC:
-
Deep layers of the superior colliculus
- dPAG:
-
Dorsal columns of the periaqueductal grey matter
- EM-1:
-
Endomorphin-1
- GABA:
-
Gamma-aminobutyric acid
- GPCR:
-
G protein-coupled nociceptin receptor
- NalBzoH:
-
Naloxone benzoylhydrazone
- N/OFQ:
-
Nociceptin/orphanin FQ
- ORL1/NOP:
-
Nociceptin receptor
- PAG:
-
Periaqueductal grey matter
- SNpr:
-
Substantia nigra pars reticulata
References
Almada RC, Coimbra NC (2015) Recruitment of striatonigral disinhibitory and nigrotectal inhibitory GABAergic pathways during the organization of defensive behavior by mice in a dangerous environment with the venomous snake Bothrops alternatus (Reptilia, Viperidae). Synapse 69:299–313
Anton B, Fein J, To T, Li X, Silberstein L, Evans CJ (1996) Immunohistochemical localization of ORL-1 in the central nervous system of the rat. J Comp Neurol 368:229–251
Asakawa A, Inui A, Momose K, Ueno N, Fujino MA, Kasuga M (1998) Endomorphins have orexigenic and anxiolytic activities in mice. Neuroreport 10:2265–2267
Bignan GC, Battista K, Connolly PJ, Orsini MJ, Liu J, Middleton SA, Reitz AB (2006) 3-(4-Piperidinyl)indoles and 3-(4-piperidinyl)pyrrolo-[2,3-b]pyridines as ligands for the ORL-1 receptor. Bioorg Med Chem Lett 13:3524–3528
Bigoni R, Cao G, Rizzi A, Okawa H, Regoli D, Smart D, Lambert DG (2002) Effects of naloxone benzoylhydrazone on native and recombinant nociceptin/orphanin FQ receptors. Can J Physiol Pharmacol 80:407–412
Bolam JP, Hanley JJ, Booth PA, Bevan MD (2000) Synaptic organization of the basal ganglia. J Anat 196:527–542
Borelli KG, Nobre NJ, Brandão ML, Coimbra NC (2004) Effects of acute and chronic fluoxetine and diazepam on freezing behavior induced by electrical stimulation of dorsolateral and lateral columns of the periaqueductal gray matter. Pharmacol Biochem Behav 77:557–566
Brandão ML, Anseloni VZ, Pandóssio JE, De Araújo JE, Castilho VM (1999) Neurochemical mechanisms of the defensive behavior in the dorsal midbrain. Neurosci Biobehav Rev 23:863–875
Bunzow JR, Saez C, Mortrud M, Bouvier C, Williams JT, Low M, Grandy DK (1994) Molecular cloning and tissue distribution of a putative member of the rat opioid receptor gene family that is not a mu, delta or kappa opioid receptor type. FEBS Lett 347(2–3):284–288
Calvo F, Coimbra NC (2006) Interactions between opioid-peptide-containing pathways and GABAA-receptor-mediated systems modulate panic-like-induced behaviors elicited by electric and chemical stimulation of the inferior colliculus. Brain Res 1104:92–102
Cardoso SH, Melo L, Coimbra NC, Brandão ML (1992) Opposite effects of low and high doses of morphine on neural substrates of aversion in the inferior colliculus. Behav Pharmacol 3:489–495
Castellan-Baldan L, da Costa Kawasaki M, Ribeiro SJ, Calvo F, Corrêa VMA, Coimbra NC (2006) Topographic and functional neuroanatomical study of GABAergic disinhibitory striatum-nigral inputs and inhibitory nigrocollicular pathways: neural hodology recruiting the substantia nigra, pars reticulata, for the modulation of the neural activity in the inferior colliculus involved with panic- like emotions. J Chem Neuroanat 32:1–27
Chevalier G, Thierry AM, Shibasaki T, Feger J (1981) Evidence for a GABAergic inhibitory nigro-collicular pathway in the rat. Neurosci Lett 21:67–70
Coimbra NC, Leão Borges PC, Brandão ML (1989) GABAergic fibers from substantia nigra, pars reticulata, modulate escape behaviour induced by midbrain central gray stimulation. Braz J Med Biol Res 22:111–114
Coimbra NC, Tomaz C, Brandão ML (1992) Evidence for the involvement of serotonin in the antinociception induced by electrical or chemical stimulation of the mesencephalic tectum. Behav Brain Res 28:1–2
Coimbra NC, Brandão ML (1993) GABAergic nigro-collicular pathways modulate the defensive behaviour elicited by midbrain tectum stimulation. Behav Brain Res 59:131–139
Coimbra NC, Eichenberger GCD, Gorchinski RT, Maisonnette S (1996) Effects of opioid receptor blockade on defensive reactions elicited by electrical stimulation within the deep layers of the superior colliculus and DPAG. Brain Res 736:348–352
Coimbra NC, Osaki MY, Eichenberger GCD, Ciscato JG Jr, Jucá CEB, Biojone CR (2000) Effects of opioid receptor blockade on defensive behavior elicited by electrical stimulation of the aversive substrates of the inferior colliculus in Rattus norvegicus (Rodentia, Muridae). Psychopharmacology 152:422–430
Coimbra NC, De Oliveira R, Freitas RL, Ribeiro SJ, Borelli KG, Pacagnella RC, Moreira JE, da Silva LA, Melo LL, Lunardi LO, Brandão ML (2006) Neuroanatomical approaches of the tectum-reticular pathways and immunohistochemical evidence for serotonin-positive perikarya on neuronal substrates of the superior colliculus and periaqueductal gray matter involved in the elaboration of the defensive behavior and fear-induced analgesia. Exp Neurol 197:93–112
Coimbra NC, Calvo F, Almada RC, Freitas RL, Paschoalin-Maurin T, dos Anjos-Garcia T, Elias-Filho DH, Ubiali WA, Lobão-Soares B, Tracey I (2017a) Opioid neurotransmission modulates defensive behaviour and fear-induced antinociception in dangerous situations. Neuroscience 354:178–195
Coimbra NC, Paschoalin-Maurin T, Bassi GS, Kanashiro A, Biagioni AF, Felippotti TT, Elias-Filho DH, Mendes-Gomes J, Cysne-Coimbra JP, Almada RC, Lobão-Soares B (2017b) Critical neuropsychobiological analysis of panic attack- and anticipatory anxiety-like behaviors in rodents confronted with snakes in polygonal arenas and complex labyrinths: a comparison to the elevated plus- and T-maze behavioral tests. Rev Bras Psiquiatr 39:72–83
Da Silva JA, De Freitas RL, Eichenberger GCD, Padovan CM, Coimbra NC (2013) Chemical neuroanatomical and psychopharmacological evidence that κ receptor-mediated endogenous opioid peptide neurotransmission in the dorsal and ventral mesencephalon modulates panic-like behaviour. Eur J Pharmacol 698:235–245
Da Silva JA, Biagioni AF, Almada RC, de Souza Crippa JA, Cecílio Hallak JE, Zuardi AW, Coimbra NC (2015) Dissociation between the panicolytic effect of cannabidiol microinjected into the substantia nigra, pars reticulata, and fear-induced antinociception elicited by bicuculline administration in deep layers of the superior colliculus: The role of CB1-cannabinoid receptor in the ventral mesencephalon. Eur J Pharmacol 758:153–63
Deniau JM, Feger J, Le Guyader C (1976) Striatal evoked inhibition of identified nigro-thalamic neurons. Brain Res 104:152–156
Eichenberger GCD, Ribeiro SJ, Osaki MY, Maruoka RY, Resende GCC, Castellan-Baldan L, Corrêa SAL, Da Silva LA, Coimbra NC (2002) Neuroanatomical and psychopharmacological evidence for interaction between opioid and GABAergic neuronal pathways in the modulation of fear and defense elicited by electrical and chemical stimulation of deep layers of the superior colliculus and dorsal periqueductal gray matter. Neuropharmacology 42:48–59
Florin S, Suaudeau C, Meunier JCE, Costentin J (1996) Nociceptin stimulates locomotion and exploratory behaviour in mice. Eur J Pharmacol 1:9–13
Fukuda K, Kato S, Mori K, Nishi M, Takeshima H, Iwabe N, Miyata T, Houtani T, Sugimoto T (1994) cDNA cloning and regional distribution of a novel member of the opioid receptor family. FEBS Lett 343:42–46
Gavioli EC, Vaughan CW, Marzola G, Guerrini R, Mitchell VA, Zucchini S, De Lima TCM, Rae GA, Salvadori S, Regoli D, Calo’ G (2004) Antidepressant-like effects of the nociceptin/orphanin FQ receptor antagonist UFP-101: new evidences in rats and mice. Naunyn Schmiedebergs Arch Pharmacol 369:547–553
Goeldner C, Reiss D, Wichmann J, Kieffer BL, Ouagazzal AM (2009) Activation of nociceptin opioid peptide (NOP) receptor impairs contextual fear learning in mice through glutamatergic mechanisms. Neurobiol Learn Mem 91:393–401
Gonzalez-Hernandez T, Rodriguez M (2000) Compartmental organization and chemical profile of dopaminergic and GABAergic neurons in the substantia nigra of the rat. J Comp Neurol 421:107–135
Grofová I, Ottersen OP, Rinvik E (1978) Mesencephalic and diencephalic afferents to the superior colliculus and periaqueductal gray substance demonstrated by retrograde axonal transport of horseradish peroxidase in the cat. Brain Res 146:205–220
Gulya K, Kriván M, Nyolczas N, Sarnyai Z, Kovács GL (1988) Central effects of the potent and highly selective mu opioid antagonist D-Phe-Cys-Tyr-D-Trp-Orn-Thr-Pen-Thr-NH2 (CTOP) in mice. Eur J Pharmacol 3:355–360
Hawkins KN, Knapp RJ, Lui GK, Gulya K, Kazmierski W, Wan YP, Pelton JT, Hruby VJ, Yamamura HI (1989) [3H]-[H-D-Phe-Cys-Tyr-D-Trp-Orn-Thr-Pen-Thr-NH2] ([3H]CTOP), a potent and highly selective peptide for mu opioid receptors in rat brain. J Pharmacol Exp Ther 248:73–80
Jayaraman A, Batton RR 3rd, Carpenter MB (1977) Nigrotectal projections in the monkey: an autoradiographic study. Brain Res 135:147–152
Kallupi M, Oleata CS, Luu G, Teshima K, Ciccocioppo R, Roberto M (2014) MT-7716, a novel selective nonpeptidergic NOP receptor agonist, effectively blocks ethanol-induced increase in GABAergic transmission in the rat central amygdala. Front Integr Neurosci 18:8–18
Kalyuzhny AE, Dooyema J, Wessendorf MW (2000) Opioid and GABA(A)-receptors are co-expressed by neurons in rat brain. Neuroreport 11:2625–2628
Loughlin SE, Massamiri TR, Kornblum HI, Leslie FM (1985) Postnatal development of opioid systems in rat brain. Neuropeptides 5:469–472
Maisonnette SS, Kawasaki MC, Coimbra NC, Brandão ML (1996) Effects of lesions of amygdaloid nuclei and substantia nigra on aversive responses induced by electrical stimulation of the inferior colliculus. Brain Res Bull 40:93–98
Mamiya T, Noda Y, Nishi M, Takeshima H, Nabeshima T (1999) Nociceptin system plays a role in the memory retention: involvement of naloxone benzoylhydrazone binding sites. Neuroreport 10:1171–1175
Mansour A, Fox CA, Burke S, Akil H, Watson SJ (1995) Immunohistochemical localization of the cloned mu opioid receptor in the rat CNS. J Chem Neuroanat 8:283–305
Mansour A, Burke S, Pavlic RJ, Akil H, Watson S (1996) Immunohistochemical localization of the cloned kappa 1 receptor in the rat CNS and pituitary. J Neurosci 71:671–690
Marti M, Stocchi S, Paganini F, Mela F, De Risi C, Calo’ G, Guerrini R, Barnes TA, Lambert DG, Beani L, Bianchi C, Morari M (2003) Pharmacological profiles of presynaptic nociceptin/orphanin FQ receptors modulating 5-hydroxytryptamine and noradrenaline release in the rat neocortex. Br J Pharmacol 1:91–98
Marti M, Viaro R, Guerrini R, Franchi G, Morari M (2009) Nociceptin/orphanin FQ modulates motor behavior and primary motor cortex output through receptors located in substantia nigra reticulata. Neuropsychopharmacology 34:341–355
Miller-Pérez C, Sánchez-Islas E, Pellicer F, Rodríguez-Manzo G, Cruz SL, León-Olea M (2008) Role of nociceptin/orphanin FQ and the pseudopeptide [Phe1Psi(CH2NH)Gly2]-nociceptin(1-13)-NH2 and their interaction with classic opioids in the modulation of thermonociception in the land snail Helix aspersa. Eur J Pharmacol 581:77–85
Mollereau C, Parmentier M, Mailleux P, Butour JL, Moisand C, Chalon P, Caput D, Vassart G, Meunier JC (1994) ORL1, a novel member of the opioid receptor family. Cloning, functional expression and localization. FEBS Lett 341:33–38
Nazzaro C, Rizzi A, Salvadori S, Guerrini R, Regoli D, Zeilhofer HU, Calo G (2007) UFP-101 antagonizes the spinal antinociceptive effects of nociceptin/orphanin FQ: behavioral and electrophysiological studies in mice. Peptides 3:663–669
Neal CR Jr, Mansour A, Reinscheid R, Nothacker HP, Civelli O, Akil H, Watson SJ Jr (1999) Opioid receptor-like (ORL1) receptor distribution in the rat central nervous system: comparison of ORL1 receptor mRNA expression with (125) I-[(14)Tyr]-orphanin FQ binding. J Comp Neurol 4:563–605
Nishi M, Houtani T, Noda Y, Mamiya T, Sato K, Doi T, Kuno J, Takeshima H, Nukada T, Nabeshima T, Yamashita T, Noda TE, Sugimoto T (1997) Unrestrained nociceptive response and disregulation of hearing ability in mice lacking the nociceptin/orphaninFQ receptor. EMBO J 8:1858–1864
Nobre MJ, Ribeiro Dos Santos N, Aguiar MS, Brandão ML (2000) Blockade of mu- and activation of kappa-opioid receptors in the dorsal periaqueductal gray matter produce defensive behavior in rats tested in the elevated plus-maze. Eur J Pharmacol 1-2:145–151
Noda Y, Mamiya T, Nabeshima T, Nishi M, Higashioka M, Takeshima H (1998) Loss of antinociception induced by naloxone benzoylhydrazone in nociceptin receptor-knockout mice. J Biol Chem 273:18047–18051
Osaki MY, Castellan-Baldan L, Calvo F, Carvalho AD, Felippotti TT, de Oliveira R, Ubiali WA, Paschoalin-Maurin T, Elias-Filho DH, Motta V, da Silva LA, Coimbra NC (2003) Neuroanatomical and neuropharmacological study of opioid pathways in the mesencephalic tectum: effect of μ1- and κ-opioid receptor blockade on escape behavior induced by electrical stimulation of the inferior colliculus. Brain Res 992:179–192
Paul D, Levison JA, Howard DH, Pick CG, Hahn EF, Pasternak GW (1990) Naloxone benzoylhydrazone (NalBzoH) analgesia. J Pharmacol Exp Ther 2:769–774
Paxinos G, Watson C (2007) The rat brain in stereotaxic coordinates, 6th edn. Academic, San Diego
Post A, Smart TS, Krikke-Workel J, Dawson GR, Harmer CJ, Browning M, Jackson K, Kakar R, Mohs R, Statnick M, Wafford K, McCarthy A, Barth V, Witkin JM (2016) A selective nociceptin receptor antagonist to treat depression: evidence from preclinical and clinical studies. Neuropsychopharmacology 41:1803–1812
Reinscheid RK, Nothacker HP, Bourson A, Ardati A, Henningsen RA, Bunzow JR, Grandy DK, Langen H, Monsma FJ, Civelli O (1995) Orphanin FQ: a neuropeptide that activates an opioid like G protein-coupled receptor. Science 270:792–794
Ribeiro SJ, Ciscato JG Jr, de Oliveira R, de Oliveira RC, d’Ângelo-Dias R, Carvalho AD, Felippotti TT, Rebouças ECC, Castellan-Baldan L, Hoffmann A, Corrêa SAL, Moreira JE, Coimbra NC (2005) Functional and ultrastructural neuroanatomy of interactive intratectal/tectonigral mesencephalic opioid inhibitory links and nigrotectal GABAergic pathways: Involvement of GABAA and μ1-opioid receptors in the modulation of panic-like reactions elicited by electrical stimulation of the dorsal midbrain. J Chem Neuroanat 30:184–200
Sante AB, Nobre MJ, Brandão ML (2000) Place aversion induced by blockade of mu or activation of kappa opioid receptors in the dorsal periaqueductal gray matter. Behav Pharmacol 7-8:583–589
Schlicker E, Morari M (2000) Nociceptin/orphanin FQ and neurotransmitter release in the central nervous system. Peptides 21:1023–1029
Schmitt P, Di Scala G, Brandão ML, Karli P (1985) Behavioral effects of microinjections of SR 95103, a new GABAA antagonist, into the medial hypothalamus or the mesencephalic central gray. Eur J Pharmacol 117:149–158
Simmonds MA (1980) Evidence that bicuculline and picrotoxin act at separate sites to antagonize gamma-aminobutyricacid in rat cuneate nucleus. Neuropharmacology 1:39–45
Suvorov NF, Voilokova NL, Gorbachevskaya AI, Mikhailov AV (1997) Striato-nigro-thalamic mechanisms in the organization of behavior. Neurosci Behav Physiol 27:59–67
Thomsen C, Hohlweg R (2000) (8-Naphthalen-1-ylmethyl-4-oxo-1-phenyl-1,3,8-triaza-spiro[4. 5]dec-3-yl)-acetic acid methyl ester (NNC 63-0532) is a novel potent nociceptin receptor agonist. Br J Pharmacol 5:903–908
Tongjaroenbungam W, Jongkamonwiwat N, Cunningham J, Phansuwan-Pujito P, Dodson HC, Forge A, Govitrapong P, Casalotti SO (2004) Opioid modulation of GABA release in the rat inferior colliculus. BMC Neurosci 5:31–36
Tongjaroenbuangam W, Jongkamonwiwat N, Phansuwan-Pujito P, Casalotti SO, Forge A, Dodson H, Govitrapong P (2006) Relationship of opioid receptors with GABAergic neurons in the rat inferior colliculus. Eur J Neurosci 24:1987–1994
Vaccarino AL, Olson GA, Olson RD, Kastin AJ (1999) Endogenous opiates. Peptides 12:1527–1574
Vitale G, Ruggieri V, Filaferro M, Frigeri C, Alboni S, Tascedda F, Brunello N, Guerrini R, Cifani C, Massi M (2009) Chronic treatment with the selective NOP receptor antagonist [Nphe 1, Arg 14, Lys 15]N/OFQ-NH 2 (UFP-101) reverses the behavioural and biochemical effects of unpredictable chronic mild stress in rats. Psychopharmacology 207:173–189
Wang JB, Johnson PS, Imai Y, Persico AM, Ozenberger BA, Eppler CM, Uhl GR (1994) cDNA cloning of an orphan opiate receptor gene family member and its splice variant. FEBS Lett 348:75–79
Wang H, DuBois DW, Tobery AN, Griffith WH, Brandt P, Frye GD (2013) Long-lasting distortion of GABA signaling in MS/DB neurons after binge-like ethanol exposure during initial synaptogenesis. Brain Res 1520:36–50
Witkin JM, Statnick MA, Rorick-Kehn LM, Pintar JE, Ansonoff M, Chen Y, Tucker RC, Ciccocioppo R (2014) The biology of nociceptin/orphanin FQ (N/OFQ) related to obesity, stress, anxiety, mood, and drug dependence. Pharmacol Ther 1413:283–299
Wolfarth S, Dulska E, Harasiewicz A (1977) The participation of the nigro-thalamic pathway in the nigral control of the caudate nucleus. Pol J Pharmacol Pharm 29:49–60
Yamada H, Nakamoto H, Suzuki Y, Ito T, Aisaka K (2002) Pharmacological profiles of a novel opioid receptor-like1 (ORL1) receptor antagonist, JTC-801. Br J Pharmacol 2:323–332
Yu Y, Wang X, Cui Y, Fan YZ, Liu J, Wang R (2006) Abnormal modulation of cholinergic neurotransmission by endomorphin 1 and endomorphin 2 in isolated bronchus of type 1 diabetic rats. Peptides 11:2770–2777
Zadina JE, Hackler L, Ge LJ, Kastin AJ (1997) A potent and selective endogenous agonist for the mu-opiate receptor. Nature 6624:499–502
Acknowledgements
This study was supported by CNPq (process 483763/2010-1), FAEPA (processes 70/2002, 210/2005, and 345/2009), and FAPESP (processes 1995/3604-4, 1995/8418-4, 2007/01174-1, 2009/54014-7, 2012/03798-0, and 2017/11855-8). J.A. Silva was a recipient of a Scientific Initiation scholarship from CNPq (PIBIC process 2005.1.891.17.3) and was also supported by FAPESP and CNPq post-graduation fellowships (CNPq M.Sc. process 130170/2009-7; FAPESP M.Sc. process 2009/02458-9; CNPq Sc.D. process 142844/2011-0; FAPESP post-doctoral process 2015/10313-1). A.F. Biagioni was supported by FAPESP (Sc.D. process 2010/15140-4; post-doctorate process 2014/10742-7). R.C. Almada is a post-doctoral student supported by FAPESP (process 2012/22681-7). R.L. de Freitas was supported by FAPESP (Scientific Initiation Scholarship 01/03752-6, M.Sc. fellowship 03/05256-1, post-doctoral fellowship 2009/17258-5, Young Researchers in Emergent Centres Program fellowship 2013/12916-0, and research grant 2014/11869-0) and CAPES (Sc.D. Fellowship). Coimbra was granted a research fellowship (level 1A) from CNPq (processes 301905/2010-0 and 301341/2015-0). The authors are grateful to D.H. Elias-Filho for the expert technical assistance. D.H. Elias-Filho received a technician scholarship from FAPESP (TT-2, process 02/01497-1) and was the recipient of scholarships sponsored by CNPq (processes 501858/2005-9, 500896/2008-9, and 505461/2010-2).
Author information
Authors and Affiliations
Contributions
JA Silva performed the experiments, analysed and interpreted the data, wrote the manuscript, and designed the figures. AF Biagioni performed the experiments and analysed and interpreted the data. RC Almada analysed the data, designed the figures, and revised the manuscript. RL de Freitas performed the experiments, analysed the data, and revised the manuscript. NC Coimbra designed the experiments, analysed and interpreted the data, wrote the manuscript and approved the final manuscript. All authors have approved the final version of the manuscript.
Corresponding author
Ethics declarations
All experiments were performed in accordance with the recommendations of the Brazilian Society for Neuroscience and Behaviour (SBNeC). The study was approved by the FMRP-USP Ethics in Animal Research Committee (CETEA; process 112/2011).
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
da Silva, J.A., Biagioni, A.F., Almada, R.C. et al. Panicolytic-like effects caused by substantia nigra pars reticulata pretreatment with low doses of endomorphin-1 and high doses of CTOP or the NOP receptors antagonist JTC-801 in male Rattus norvegicus . Psychopharmacology 234, 3009–3025 (2017). https://doi.org/10.1007/s00213-017-4678-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-017-4678-6