Abstract
Rationale
Elevated dopamine function is thought to play a key role in both the rewarding effects of addictive drugs and the pathophysiology of schizophrenia. Accumulating epidemiological evidence indicates that cannabis use is a risk factor for the development of schizophrenia. However, human neurochemical imaging studies that examined the impact of ∆9-tetrahydrocannabinol (THC), the main psychoactive component in cannabis, on striatal dopamine release have provided inconsistent results.
Objectives
The objective of this study is to assess the effect of a THC challenge on human striatal dopamine release in a large sample of healthy participants.
Methods
We combined human neurochemical imaging data from two previous studies that used [11C]raclopride positron emission tomography (PET) (n = 7 and n = 13, respectively) to examine the effect of THC on striatal dopamine neurotransmission in humans. PET images were re-analysed to overcome differences in PET data analysis.
Results
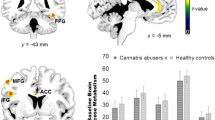
THC administration induced a significant reduction in [11C]raclopride binding in the limbic striatum (−3.65 %, from 2.39 ± 0.26 to 2.30 ± 0.23, p = 0.023). This is consistent with increased dopamine levels in this region. No significant differences between THC and placebo were found in other striatal subdivisions.
Conclusions
In the largest data set of healthy participants so far, we provide evidence for a modest increase in human striatal dopamine transmission after administration of THC compared to other drugs of abuse. This finding suggests limited involvement of the endocannabinoid system in regulating human striatal dopamine release and thereby challenges the hypothesis that an increase in striatal dopamine levels after cannabis use is the primary biological mechanism underlying the associated higher risk of schizophrenia.

Similar content being viewed by others
Abbreviations
- BPND :
-
Non-displaceable binding potential
- DVR:
-
Distribution volume ratio
- PET:
-
Positron emission tomography
- ROI:
-
Region of interest
- SPECT:
-
Single-photon emission computed tomography
- THC:
-
∆9-Tetrahydrocannabinol
References
Agurell S, Halldin M, Lindgren JE, Ohlsson A, Widman M, Gillespie H, Hollister L (1986) Pharmacokinetics and metabolism of delta 1-tetrahydrocannabinol and other cannabinoids with emphasis on man. Pharmacol Rev 38:21–43
Arseneault L, Cannon M, Witton J, Murray RM (2004) Causal association between cannabis and psychosis: examination of the evidence. Br J Psychiatry 184:110–117
Barkus E, Morrison PD, Vuletic D, Dickson J, Ell PJ, Pilowsky LS, Brenneisen R, Holt DW, Powell J, Kapur S, Murray RM (2011) Does intravenous delta9-tetrahydrocannabinol increase dopamine release? A SPET study. J Psychopharmacol 25:1462–1468
Bloomfield MA, Morgan CJ, Egerton A, Kapur S, Curran HV, Howes OD (2014) Dopaminergic function in cannabis users and its relationship to cannabis-induced psychotic symptoms. Biol Psychiatry 75:470–478
Boileau I, Assaad JM, Pihl RO, Benkelfat C, Leyton M, Diksic M, Tremblay RE, Dagher A (2003) Alcohol promotes dopamine release in the human nucleus accumbens. Synapse 49:226–231
Bossong MG, van Berckel BN, Boellaard R, Zuurman L, Schuit RC, Windhorst AD, van Gerven JMA, Ramsey NF, Lammertsma AA, Kahn RS (2009) Delta 9-tetrahydrocannabinol induces dopamine release in the human striatum. Neuropsychopharmacology 34:759–766
Breier A, Su TP, Saunders R, Carson RE, Kolachana BS, De Bartolomeis A, Weinberger DR, Weisenfeld N, Malhotra AK, Eckelman WC, Pickar D (1997) Schizophrenia is associated with elevated amphetamine-induced synaptic dopamine concentrations: evidence from a novel positron emission tomography method. Proc Natl Acad Sci U S A 94:2569–2574
Brody AL, Mandelkern MA, Olmstead RE, Allen-Martinez Z, Scheibal D, Abrams AL, Costello MR, Farahi J, Saxena S, Monterosso J, London ED (2009) Ventral striatal dopamine release in response to smoking a regular vs a denicotinized cigarette. Neuropsychopharmacology 34:282–289
Cardenas L, Houle S, Kapur S, Busto UE (2004) Oral D-amphetamine causes prolonged displacement of [11C]raclopride as measured by PET. Synapse 51:27–31
Carson RE, Breier A, de Bartolomeis A, Saunders RC, Su TP, Schmall B, Der MG, Pickar D, Eckelman WC (1997) Quantification of amphetamine-induced changes in [11C]raclopride binding with continuous infusion. J Cereb Blood Flow Metab 17:437–447
Chen JP, Paredes W, Li J, Smith D, Lowinson J, Gardner EL (1990) Delta 9-tetrahydrocannabinol produces naloxone-blockable enhancement of presynaptic basal dopamine efflux in nucleus accumbens of conscious, freely-moving rats as measured by intracerebral microdialysis. Psychopharmacology (Berl) 102:156–162
D’Souza DC, Braley G, Blaise R, Vendetti M, Oliver S, Pittman B, Ranganathan M, Bhakta S, Zimolo Z, Cooper T, Perry E (2008) Effects of haloperidol on the behavioral, subjective, cognitive, motor, and neuroendocrine effects of Delta-9-tetrahydrocannabinol in humans. Psychopharmacology (Berl) 198:587–603
El Khoury MA, Gorgievski V, Moutsimilli L, Giros B, Tzavara ET (2012) Interactions between the cannabinoid and dopaminergic systems: evidence from animal studies. Prog Neuropsychopharmacol Biol Psychiatry 38:36–50
Fadda P, Scherma M, Spano MS, Salis P, Melis V, Fattore L, Fratta W (2006) Cannabinoid self-administration increases dopamine release in the nucleus accumbens. Neuroreport 17:1629–1632
French ED (1997) Delta9-tetrahydrocannabinol excites rat VTA dopamine neurons through activation of cannabinoid CB1 but not opioid receptors. Neurosci Lett 226:159–162
French ED, Dillon K, Wu X (1997) Cannabinoids excite dopamine neurons in the ventral tegmentum and substantia nigra. Neuroreport 8:649–652
Gardner EL (2005) Endocannabinoid signaling system and brain reward: emphasis on dopamine. Pharmacol Biochem Behav 81:263–284
Gessa GL, Melis M, Muntoni AL, Diana M (1998) Cannabinoids activate mesolimbic dopamine neurons by an action on cannabinoid CB1 receptors. Eur J Pharmacol 341:39–44
Howes OD, Kapur S (2014) A neurobiological hypothesis for the classification of schizophrenia: type A (hyperdopaminergic) and type B (normodopaminergic). Br J Psychiatry 205:1–3
Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, Holden J, Houle S, Huang SC, Ichise M, Iida H, Ito H, Kimura Y, Koeppe RA, Knudsen GM, Knuuti J, Lammertsma AA, Laruelle M, Logan J, Maguire RP, Mintun MA, Morris ED, Parsey R, Price JC, Slifstein M, Sossi V, Suhara T, Votaw JR, Wong DF, Carson RE (2007) Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab 27:1533–1539
Lammertsma AA, Bench CJ, Hume SP, Osman S, Gunn K, Brooks DJ, Frackowiak RS (1996) Comparison of methods for analysis of clinical [11C]raclopride studies. J Cereb Blood Flow Metab 16:42–52
Laruelle M, Iyer RN, Al-Tikriti MS, Zea-Ponce Y, Malison R, Zoghbi SS, Baldwin RM, Kung HF, Charney DS, Hoffer PB, Innis RB, Bradberry CW (1997) Microdialysis and SPECT measurements of amphetamine-induced dopamine release in nonhuman primates. Synapse 25:1–14
Liem-Moolenaar M, te Beek ET, de Kam ML, Franson KL, Kahn RS, Hijman R, Touw D, van Gerven JMA (2010) Central nervous system effects of haloperidol on THC in healthy male volunteers. J Psychopharmacol 24:1697–1708
Malone DT, Taylor DA (1999) Modulation by fluoxetine of striatal dopamine release following Delta9-tetrahydrocannabinol: a microdialysis study in conscious rats. Br J Pharmacol 128:21–26
Martinez D, Slifstein M, Broft A, Mawlawi O, Hwang DR, Huang Y, Cooper T, Kegeles L, Zarahn E, Abi-Dargham A, Haber SN, Laruelle M (2003) Imaging human mesolimbic dopamine transmission with positron emission tomography. Part II: amphetamine-induced dopamine release in the functional subdivisions of the striatum. J Cereb Blood Flow Metab 23:285–300
Martinez D, Narendran R, Foltin RW, Slifstein M, Hwang DR, Broft A, Huang Y, Cooper TB, Fischman MW, Kleber HD, Laruelle M (2007) Amphetamine-induced dopamine release: markedly blunted in cocaine dependence and predictive of the choice to self-administer cocaine. Am J Psychiatry 164:622–629
Moore TH, Zammit S, Lingford-Hughes A, Barnes TR, Jones PB, Burke M, Lewis G (2007) Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet 370:319–328
Nader MA, Morgan D, Gage HD, Nader SH, Calhoun TL, Buchheimer N, Ehrenkaufer R, Mach RH (2006) PET imaging of dopamine D2 receptors during chronic cocaine self-administration in monkeys. Nat Neuroscience 9:1050–1056
Ng Cheong Ton JM, Gerhardt GA, Friedemann M, Etgen AM, Rose GM, Sharpless NS, Gardner EL (1988) The effects of delta 9-tetrahydrocannabinol on potassium-evoked release of dopamine in the rat caudate nucleus: an in vivo electrochemical and in vivo microdialysis study. Brain Res 451:59–68
Oswald LM, Wong DF, McCaul M, Zhou Y, Kuwabara H, Choi L, Brasic J, Wand GS (2005) Relationships among ventral striatal dopamine release, cortisol secretion, and subjective responses to amphetamine. Neuropsychopharmacology 30:821–832
Sevy S, Smith GS, Ma Y, Dhawan V, Chaly T, Kingsley PB, Kumra S, Abdelmessih S, Eidelberg D (2008) Cerebral glucose metabolism and D2/D3 receptor availability in young adults with cannabis dependence measured with positron emission tomography. Psychopharmacology (Berl) 197:549–56
Stokes PR, Mehta MA, Curran HV, Breen G, Grasby PM (2009) Can recreational doses of THC produce significant dopamine release in the human striatum? Neuroimage 48:186–190
Stokes PR, Egerton A, Watson B, Reid A, Lappin J, Howes OD, Nutt DJ, Lingford-Hughes AR (2012) History of cannabis use is not associated with alterations in striatal dopamine D2/D3 receptor availability. J Psychopharmacol 26:144–149
Strougo A, Zuurman L, Roy C, Pinquier JL, van Gerven JM, Cohen AF, Schoemaker RC (2008) Modelling of the concentration-effect relationship of THC on central nervous system parameters and heart rate—insight into its mechanisms of action and a tool for clinical research and development of cannabinoids. J Psychopharmacol 22:717–726
Sulcova E, Mechoulam R, Fride E (1998) Biphasic effects of anandamide. Pharmacol Biochem Behav 59:347–352
Takahashi H, Fujimura Y, Hayashi M, Takano H, Kato M, Okubo Y, Kanno I, Ito H, Suhara T (2008) Enhanced dopamine release by nicotine in cigarette smokers: a double-blind, randomized, placebo-controlled pilot study. Int J Neuropsychopharmacol 11:413–417
Tanda G, Pontieri FE, Di Chiara G (1997) Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common mu1 opioid receptor mechanism. Science 276:2048–2050
Taylor JR (1997) An Introduction to error analysis, 2nd edn. University Science Books, Sausalito, California, pp 166–168
Urban NB, Kegeles LS, Slifstein M, Xu X, Martinez D, Sakr E, Castillo F, Moadel T, O’Malley SS, Krystal JH, Abi-Dargham A (2010) Sex differences in striatal dopamine release in young adults after oral alcohol challenge: a positron emission tomography imaging study with [11C]raclopride. Biol Psychiatry 68:689–696
Urban NB, Slifstein M, Thompson JL, Xu X, Girgis RR, Raheja S, Haney M, Abi-Dargham A (2012) Dopamine release in chronic cannabis users: a [11C]raclopride positron emission tomography study. Biol Psychiatry 71:677–683
Volkow ND, Fowler JS, Wang GJ, Swanson JM (2004) Dopamine in drug abuse and addiction: results from imaging studies and treatment implications. Mol Psychiatry 9:557–569
Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F (2011) Addiction: beyond dopamine reward circuitry. Proc Natl Acad Sci U S A 108:15037–15042
Volkow ND, Wang GJ, Telang F, Fowler JS, Alexoff D, Logan J, Jayne M, Wong C, Tomasi D (2014) Decreased dopamine brain reactivity in marijuana abusers is associated with negative emotionality and addiction severity. Proc Natl Acad Sci U S A 111:E3149–E3156
Wand GS, Oswald LM, McCaul ME, Wong DF, Johnson E, Zhou Y, Kuwabara H, Kumar A (2007) Association of amphetamine-induced striatal dopamine release and cortisol responses to psychological stress. Neuropsychopharmacology 32:2310–2320
Wise RA (2009) Roles for nigrostriatal—not just mesocorticolimbic—dopamine in reward and addiction. Trends Neurosci 32:517–524
Acknowledgments
Dr. Matthijs Bossong was supported by a Rubicon grant from the Netherlands Organisation for Scientific Research. We would like to thank Dr. Ronald Boellaard and Dr. Lineke Zuurman for their help with data acquisition and analysis.
Conflict of interest
The authors declare no financial conflict of interest. The authors have full control of all primary data, and they agree to allow the journal to review their data if requested.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 160 kb)
Rights and permissions
About this article
Cite this article
Bossong, M.G., Mehta, M.A., van Berckel, B.N.M. et al. Further human evidence for striatal dopamine release induced by administration of ∆9-tetrahydrocannabinol (THC): selectivity to limbic striatum. Psychopharmacology 232, 2723–2729 (2015). https://doi.org/10.1007/s00213-015-3915-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-015-3915-0