Abstract
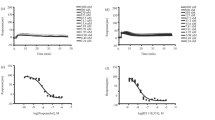
Although many β1-receptor antagonists and β2-receptor agonists have been used in pharmacotherapy for many years their pharmacological properties at all three known subtypes of β-adrenergic receptors are not always well characterized. The aim of this study was, therefore, to provide comparative binding characteristics of agonists (epinephrine, norepinephrine, isoproterenol, fenoterol, salbutamol, salmeterol, terbutalin, formoterol, broxaterol) and antagonists (propranolol, alprenolol, atenolol, metoprolol, bisoprolol, carvedilol, pindolol, BRL 37344, CGP 20712, SR 59230A, CGP 12177, ICI 118551) at all three subtypes of human β-adrenergic receptors in an identical cellular background. We generated Chinese hamster ovary (CHO) cells stably expressing the three β-adrenergic receptor subtypes at comparable levels. We characterized these receptor subtypes and analyzed the affinity of routinely used drugs as well as experimental compounds in competition binding studies, using the non-selective antagonist 125I-cyanopindolol as a radioligand. Furthermore, we analyzed the β-receptor-mediated adenylyl cyclase activity in isolated membranes from these cell lines. The results from our experiments show that all compounds exhibit distinct patterns of selectivity and activity at the three β-receptor subtypes. In particular, a number of β2- or β3-receptor agonists that are inverse agonists at the other subtypes were identified. In addition, β1-receptor antagonists with agonistic activity at β2- and β3-receptors were found. These specific mixtures of agonism, antagonism, and inverse agonism at different subtypes may have important implications for the therapeutic use of the respective compounds.


Similar content being viewed by others
References
Berkin KE, Ball SG (2001) Essential hypertension: the heart and hypertension. Heart 86:467–475
Blin N, Camoin L, Maigret B, Strosberg AD (1993) Structural and conformational features determining selective signal transduction in the β3-adrenergic receptor. Mol Pharmacol 44:1094–1104
Bouvier M, Hnatowich M, Collins S, Kobilka BK, Deblasi A, Lefkowitz RJ, Caron MG (1988) Expression of a human cDNA encoding the beta 2-adrenergic receptor in Chinese hamster fibroblasts (CHW): functionality and regulation of the expressed receptor. Mol Pharmacol 33:122–139
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brodde OE (1993) Beta-adrenoceptors in cardiac disease. Pharmacol Ther 60:405–443
Brodde OE, Michel MC (1999) Adrenergic and muscarinic receptors in the human heart. Pharmacol Rev 51:651–689
Candelore MR, Deng L, ToTa L, Xiao-Ming G, Amend A, Liu Y, Newbold R, Cascieri MA, Weber AE (1999) Potent and selective human β3-adrenergic receptor antagonists. J Pharmacol Exp Ther 290:649–655
Chen C, Okayama H (1987) High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol 7:2745–2752
Cheng Y-C, Prusoff WH (1973) Relationship between the inhibition constant (KI) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol 22:3099–3108
Conti P, Dallanoce C, De Amici M, De Micheli C, Klotz K-N (1998) Synthesis of new Δ2-isoxazoline derivatives and their pharmacological characterization as β-adrenergic receptor antagonists. Bioorg Med Chem 6:401–408
Del Carmine R, Ambrosio C, Sbraccia M, Cotecchia S, Ijzerman AP, Costa T (2002) Mutations inducing divergent shifts of constitutive activity reveal different modes of binding among catecholamine analogues to the β2-adrenergic receptor. Br J Pharmacol 135:1715–1722
De Lean A, Hancock AA, Lefkowitz RJ (1982) Validation and statistical analysis of a computer modeling method for quantitative analysis of radioligand binding data for mixtures of pharmacological receptor subtypes. Mol Pharmacol 21:5–16
Emorine LJ, Marullo S, Briend-Sutren MM, Patey G, Tate K, Delavier-Klutchko C, Strosberg AD (1989) Molecular characterization of the human beta 3-adrenergic receptor. Science 245:1118–1121
Freund S, Ungerer M, Lohse MJ (1994) A1 adenosine receptors expressed in CHO-cells couple to adenylyl cyclase and phospholipase C. Naunyn-Schmiedebergs Arch Pharmacol 350:49–56
Frielle, T, Collins S, Daniel KW, Caron MG, Lefkowitz RJ, Kobilka BK (1987) Cloning of the cDNA for the human β1-adrenergic receptor. Proc Natl Acad Sci USA 84:7290–7294
Giacobino J-P (1995) β3-Adrenoceptor: an update. Eur J Endocrinol 132:377–385
Gille E, Lemione H, Ehle B, Kaumann AJ (1985) The affinity of (-)-propranolol for β1 and β2 adrenoceptors of human heart. Differential antagonism of the positive inotropic effects and adenylate cyclase stimulation by (-)-noradrenaline and (-)-adrenaline. Naunyn-Schmiedeberg’s Arch Pharmacol 331:60–70
Guimaraes S, Moura D (2001) Vascular adrenoceptors: an update. Pharmacol Rev 53:319–356
Jakobs KH, Saur W, Schultz G (1976) Reduction of adenylyl cyclase activity in lysates of human platelets by the alpha-adrenergic component of epinephrine. J Cyclic Nucleotide Res 2:381–392
Klotz K-N, Cristalli G, Grifantini M, Vittori S, Lohse MJ (1985) Photoaffinity labeling of A1-adenosine receptors. J Biol Chem 260:14659–14664
Klotz K-N, Hessling J, Hegler J, Owman C, Kull B, Fredholm BB, Lohse MJ (1998) Comparative pharmacology of human adenosine receptor subtypes—characterization of stably transfected receptors in CHO cells. Naunyn-Schmiedebergs Arch Pharmacol 357:1–9
Kotlikoff MI, Kamm, KE (1996) Molecular mechanisms of β-adrenergic relaxation of airway smooth muscle. Annu Rev Physiol 58:115–141
Krief S, Lonnqvist F, Raimbault S, Baude B, Van Spronsen A, Arner P, Strosberg AD, Ricquier D, Emorine LJ (1993) Tissue distribution of beta 3-adrenergic receptor mRNA in man. J Clin Invest 91:344–349
Krum H (2003) Beta-blockers in chronic heart failure: what have we learned? What do we still need to know? Curr Opin Pharmacol 3:168–174
Lattion AL, Abuin L, Nenniger-Tosato M, Cotecchia S (1999) Constitutively active mutants of the β1-adrenergic receptor. FEBS Lett 457:302–306
Levin MC, Marullo S, Muntaner O, Andersson B, Magnusson Y (2002) The myocardium-protective Gly-49 variant of the β1-adrenergic receptor exhibits constitutive activity and increased desensitization and down-regulation. J Biol Chem 277:30429–30435
Liggett SB (1992) Functional properties of the rat and human β3-adrenergic receptors: differential agonist activation of recombinant receptors in Chinese Hamster Ovary cells. Mol Pharmacol 42:634–637
Lohse MJ (1992) Stable overexpression of human beta 2-adrenergic receptors in mammalian cells. Naunyn-Schmiedebergs Arch Pharmacol 345:444–451
Michel MC (1991) β-Adrenergic receptors. In: Doods HN, van Meel JCA (eds) Receptor data for biological experiments. Ellis Horwood, New York, pp 19–22
Neve KA, McGonigle P, Molinoff PB (1986) Quantitative analysis of the selectivity of radioligands for subtypes of Beta adrenergic receptors. J Pharmacol Exp Ther 238:46–53
Samama P, Pei G, Costa T, Cotecchia S, Lefkowitz RJ (1994) Negative antagonists promote an inactive conformation of the β2-adrenergic receptor. Mol Pharmacol 45:390–394
Schofield PR, Rhee LM, Peralta EG (1987) Primary structure of the human beta-adrenergic receptor gene. Nucleic Acids Res 15:3636
Steinle JJ, Booz GW, Meininger CJ, Day JNE, Granger HJ (2003) β3-Adrenergic receptor regulate retinal endothelial cell migration and proliferation. J Biol Chem 278:20681–20686
Tate KM, Briend-Sutren MM, Emorine LJ, Delavier-Klutchko C, Marullo S, Strosberg AD (1991) Expression of three human β-adrenergic-receptor subtypes in transfected Chinese hamster ovary cells. Eur J Biochem 196:357–361
Waldeck B (2002) β-Adrenoceptor agonists and asthma—100 years of development. Eur J Pharmacol 445:1–12
Whaley BS, Yuan N, Birnbaumer L, Clark RB, Barber R (1994) Differential expression of the β-adrenergic receptor modifies agonist stimulation of adenylyl cyclase: a quantitative evaluation. Mol Pharmacol 45:481–489
Acknowledgements
We thank Ms. Martina Fischer and Mr. Nico Falgner for technical assistance. This study was supported by the BIOMED 2 program “Inverse agonism. Implications for drug design” and the Leibnitz award of the Deutsche Forschungsgemeinschaft.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hoffmann, C., Leitz, M.R., Oberdorf-Maass, S. et al. Comparative pharmacology of human β-adrenergic receptor subtypes—characterization of stably transfected receptors in CHO cells. Naunyn-Schmiedeberg's Arch Pharmacol 369, 151–159 (2004). https://doi.org/10.1007/s00210-003-0860-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-003-0860-y