Abstract
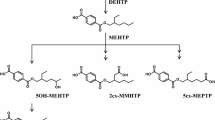
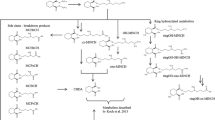
Tri-(2-ethylhexyl) trimellitate (TEHTM) is a plasticizer for PVC material and is used for medical devices as an alternative to di-(2-ethylhexyl) phthalate. As plasticizers are known to migrate easily into contact liquids, exposure of patients to TEHTM is highly probable. In the present study, human metabolism pathways of TEHTM and its elimination kinetics were investigated. For that purpose, four healthy volunteers were orally exposed to a single dose of TEHTM. TEHTM and its postulated primary metabolites were investigated in blood samples (up to 48 h after exposure), and in urine samples (collected until 72 h after exposure) using liquid chromatography tandem mass spectrometry (LC–MS/MS). TEHTM was found to be regioselectively hydrolyzed to its diesters di-2-(ethylhexyl) trimellitates (1,2-DEHTM, 2,4-DEHTM) with maximum blood concentrations at 3-h post-exposure, and to its monoester isomers mono-2-(ethylhexyl) trimellitates (1-MEHTM, 2-MEHTM) with peak blood concentrations 5-h post-exposure. For the elimination of investigated urinary metabolites, biphasic elimination kinetics was observed. The most dominant urinary biomarker was found to be 2-MEHTM (2-mono-(2-ethylhexyl) trimellitate), followed by several specific secondary metabolites. All in all, approximately 5.8% of the orally administered dose was recovered in urine over a period of 72 h, indicating a comparatively low resorption rate of TEHTM in humans in combination with an apparently rather slow metabolism and excretion rate. In fact, TEHTM and selected metabolites were still detectable in blood and urine 48-h and 72-h post-exposure, respectively. This study is the first to elucidate TEHTM metabolism pathways in humans and to identify metabolites of TEHTM in blood and urine by usage of especially designed human biomonitoring methods. Powerful tools for exposure monitoring and risk assessment of TEHTM are therewith available for future research.






Similar content being viewed by others
References
Albro PW, Thomas R, Fishbein L (1973) Metabolism of diethylhexyl phthalate by rats-isolation and characterization of the urinary metabolites. J Chromatogr 76:321–330. https://doi.org/10.1016/S0021-9673(01)96915-8
Bernard L, Cueff R, Breysse C, Decaudin B, Sautou V, Armed Study Group (2015) Migrability of PVC plasticizers from medical devices into a simulant of infused solutions. Int J Pharm 485:341–347. https://doi.org/10.1016/j.ijpharm.2015.03.030
Bourdeaux D, Yessaad M, Chennell P, Larbre V, Eljezi T, Bernard L, Sautou V, Armed Study Group (2016) Analysis of PVC plasticizers in medical devices and infused solutions by GC-MS. J Pharm Biomed Anal 118:206–213. https://doi.org/10.1016/j.jpba.2015.10.034
Chiellini F, Ferri M, Morelli A, Dipaola L, Latini G (2013) Perspectives on alternatives to phthalate plasticized poly(vinyl chloride) in medical devices applications. Prog Polym Sci 38:1067–1088. https://doi.org/10.1016/j.progpolymsci.2013.03.001
Daniel JW, Bratt H (1974) The absorption, metabolism and tissue distribution of di(2-ethylhexyl) phthalate in rats. Toxicology 2:51–65
Derendorf H, Gramatté T, Schäfer HG (2002) Einführung in die Theorie und Relevanz für die Arzneimitteltherapie, 2 edn. Wissenschaftliche Verlagsgesellschaft mbH, Stuttgart
Derendorf H, Gramatté T, Schäfer HG, Staab A (2011) Pharmakokinetik kompakt, 3 edn. Wissenschaftliche Verlagssgesellschaft mbH, Stuttgart
ECHA (European Chemicals Agency) (2017a) Information on registered substances. Dataset on tris(2-ethylhexyl) benzene-1,2,4-tricarboxylate (CAS Number 3319-31-1)
ECHA (European Chemicals Agency) (2017b) Registration dossier on tris(2-ethylhexyl) benzene-1,2,4-tricarboxylate (CAS Number 3319-31-1)
Eckert E, Münch F, Göen T, Purbojo A, Müller J, Cesnjevar R (2016) Comparative study on the migration of di-2-ethylhexyl phthalate (DEHP) and tri-2-ethylhexyl trimellitate (TOTM) into blood from PVC tubing material of a heart-lung machine. Chemosphere 145:10–16. https://doi.org/10.1016/j.chemosphere.2015.11.067
EFSA (European Food Safety Authority) (2005) Opinion of the scientific panel on food additives, flavourings, processing aids and materials in contact with food (AFC) on a request from the commission related to bis(2-ethylhexyl)phthalate (DEHP) for use in food contact materials. EFSA J 243:1–20. https://doi.org/10.2903/j.efsa.2005.243
Eljezi T, Pinta P, Richard D, Pinguet J, Chezal JM, Chagnon MC, Sautou V, Grimandi G, Moreau E (2017) In vitro cytotoxic effects of DEHP-alternative plasticizers and their primary metabolites on a L929 cell line. Chemosphere 173:452–459. https://doi.org/10.1016/j.chemosphere.2017.01.026
Enriquez PM, Giordano CJ, DiVincenzo GD (1984) Absorption and metabolism of hexyl-2-14C-tri(2-ethylhexyl) trimellitate in the rat. US Environmental Protection Agency, Washington DC, pp 1–136
Green R, Hauser R, Calafat AM, Weuve J, Schettler T, Ringer S, Huttner K, Hu H (2005) Use of di(2-ethylhexyl) phthalate-containing medical products and urinary levels of mono(2-ethylhexyl) phthalate in neonatal intensive care unit infants. Environ Health Perspect 113:1222–1225. https://doi.org/10.1289/ehp.7932
Grillo M, Hensley T, Lam TT, Li Y, Loewen GJ, Nassar AF, Ogilvie BW, Paris B, Parkinson A, Slatter JG (2010) Biotransformation and metabolites elucidation of xenobiotics: characterization and identification. Wiley, Hoboken
Heudorf U, Mersch-Sundermann V, Angerer J (2007) Phthalates: toxicology and exposure. Int J Hyg Environ Health 210:623–634. https://doi.org/10.1016/j.ijheh.2007.07.011
Hodgson J (1987) Results of peroxisome induction studies on tri (2-ethylhexyl) trimellitate and 2-ethylhexanol. Toxicol Ind Health 3:49–61. https://doi.org/10.1177/074823378700300205
Höllerer C, Müller J, Göen T, Eckert E (2017) Isomeric separation and quantitation of di-(2-ethylhexyl) trimellitates and mono-(2-ethylhexyl) trimellitates in blood by LC–MS/MS. J Chromatogr B 1061–1062:153–162. https://doi.org/10.1016/j.jchromb.2017.07.014
Höllerer C, Becker G, Göen T, Eckert E (2018a) Regioselective ester cleavage of di-(2-ethylhexyl) trimellitates by porcine liver esterase. Toxicol In Vitro 47:178–185. https://doi.org/10.1016/j.tiv.2017.11.015
Höllerer C, Göen T, Eckert E (2018b) Comprehensive monitoring of specific metabolites of tri-(2-ethylhexyl) trimellitate (TEHTM) by column-switching liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem 410:4343–4357. https://doi.org/10.1007/s00216-018-1086-7
Kambia K, Dine T, Azar R, Gressier B, Luyckx M, Brunet C (2001) Comparative study of the leachability of di(2-ethylhexyl) phthalate and tri(2-ethylhexyl) trimellitate from haemodialysis tubing. Int J Pharm 229:139–146. https://doi.org/10.1016/S0378-5173(01)00840-7
Kambia K, Dine T, Gressier B, Dupin-Spriet T, Luyckx M, Brunet C (2004) Evaluation of the direct toxicity of trioctyltrimellitate (TOTM), di(2-ethylhexyl) phthalate (DEHP) and their hydrolysis products on isolated rat hepatocytes. Int J Artif Organs 27:971–978. https://doi.org/10.1177/039139880402701110
Kato K, Silva MJ, Needham LL, Calafat AM (2005) Determination of 16 phthalate metabolites in urine using automated sample preparation and on-line preconcentration/high-performance liquid chromatography/tandem mass spectrometry. Anal Chem 77:2985–2991. https://doi.org/10.1021/ac0481248
Kavlock R, Boekelheide K, Chapin R, Cunningham M, Faustman E, Foster P, Golub M, Henderson R, Hinberg I, Little R, Seed J, Shea K, Tabacova S, Tyl R, Williams P, Zacharewski T (2002) NTP center for the evaluation of risks to human reproduction: phthalates expert panel report on the reproductive and developmental toxicity of di(2-ethylhexyl) phthalate. Reprod Toxicol 16:529–653. https://doi.org/10.1016/S0890-6238(02)00032-1
Kessler W, Numtip W, Völkel W, Seckin E, Csanady GA, Putz C, Klein D, Fromme H, Filser JG (2012) Kinetics of di(2-ethylhexyl) phthalate (DEHP) and mono(2-ethylhexyl) phthalate in blood and of DEHP metabolites in urine of male volunteers after single ingestion of ring-deuterated DEHP. Toxicol Appl Pharmacol 264:284–291. https://doi.org/10.1016/j.taap.2012.08.009
Koch HM, Bolt HM, Angerer J (2004) Di(2-ethylhexyl)phthalate (DEHP) metabolites in human urine and serum after a single oral dose of deuterium-labelled DEHP. Arch Toxicol 78:123–130. https://doi.org/10.1007/s00204-003-0522-3
Koch HM, Bolt HM, Preuss R, Angerer J (2005) New metabolites of di(2-ethylhexyl)phthalate (DEHP) in human urine and serum after single oral doses of deuterium-labelled DEHP. Arch Toxicol 79:367–376. https://doi.org/10.1007/s00204-004-0642-4
Latini G (2005) Monitoring phthalate exposure in humans. Clin Chim Acta 361:20–29. https://doi.org/10.1016/j.cccn.2005.05.003
Mallow EB, Fox MA (2014) Phthalates and critically ill neonates: device-related exposures and non-endocrine toxic risks. J Perinatol 34:892–897. https://doi.org/10.1038/jp.2014.157
Martis L, Freid E, Woods E (1987) Tissue distribution and excretion of tri-(2-ethylhexyl)trimellitate in rats. J Toxicol Environ Health 20:357–366. https://doi.org/10.1080/15287398709530989
Münch F, Höllerer C, Klapproth A, Eckert E, Rüffer A, Cesnjevar R, Göen T (2018) Effect of phospholipid coating on the migration of plasticizers from PVC tubes. Chemosphere 202:742–749. https://doi.org/10.1016/j.chemosphere.2018.03.136
Ohashi A, Kotera H, Hori H, Hibiya M, Watanabe K, Murakami K, Hasegawa M, Tomita M, Hiki Y, Sugiyama S (2005) Evaluation of endocrine disrupting activity of plasticizers in polyvinyl chloride tubes by estrogen receptor alpha binding assay. J Artif Organs 8:252–256. https://doi.org/10.1007/s10047-005-0307-y
SCENIHR (Scientific Committee on Emerging and Newly-Identified Health Risks) (2015) Opinion on the safety of medical devices containing DEHP plasticized PVC or other plasticizers on neonates and other groups possibly at risk (2015 update). https://doi.org/10.2772/45179
Sigma-Aldrich (2018) GHS Material Safety Data Sheet Trioctyl trimellitate, CAS-No: 3319-31-1, Version 4.1, reviewed on 14 Jan 2015
Silva MJ, Samandar E, Preau JL Jr, Needham LL, Calafat AM (2006) Urinary oxidative metabolites of di(2-ethylhexyl) phthalate in humans. Toxicology 219:22–32. https://doi.org/10.1016/j.tox.2005.10.018
Simmchen J, Ventura R, Segura J (2012) Progress in the removal of di-[2-ethylhexyl]-phthalate as plasticizer in blood bags. Transfus Med Rev 26:27–37. https://doi.org/10.1016/j.tmrv.2011.06.001
Stroheker T, Cabaton N, Nourdin G, Regnier JF, Lhuguenot JC, Chagnon MC (2005) Evaluation of anti-androgenic activity of di-(2-ethylhexyl)phthalate. Toxicology 208:115–121. https://doi.org/10.1016/j.tox.2004.11.013
Tickner J, Schettler T, Guidotti T, McCally M, Rossi M (2001) Health risks posed by use of di-2-ethylhexyl phthalate (DEHP) in PVC medical devices: a critical review. Am J Ind Med 39:100–111. https://doi.org/10.1002/1097-0274(200101)39:13.0.CO;2-Q
Van Vliet ED, Reitano EM, Chhabra JS, Bergen GP, Whyatt RM (2011) A review of alternatives to di (2-ethylhexyl) phthalate-containing medical devices in the neonatal intensive care unit. J Perinatol 31:551–560. https://doi.org/10.1038/jp.2010.208
Wu C, Benet L (2005) Predicting drug disposition via application of BCS: transport/absorption/elimination interplay and development of a biopharmaceutics drug disposition classification system. Pharm Res 22:11–23. https://doi.org/10.1007/s11095-004-9004-4
Acknowledgements
The development of the analytical methods and the in vivo study are part of a large-scale 10-year project on the advancement of human biomonitoring in Germany. This project is a cooperation, agreed in 2010, between the German Federal Ministry for the Environment, Nature Conservation, Building and Nuclear Safety (BMUB), and the Verband der Chemischen Industrie e.V. (German Chemical Industry Association—VCI), and is managed by the German Federal Environment Agency (UBA). Experts from governmental scientific authorities, industry and science accompanied the project in selecting substances and developing methods.
Funding
The method development was financed by the Chemie Wirtschaftsförderungsgesellschaft mbH. The supply of most of the reference substances for the analytical procedures was funded by the sponsor. However, the synthesis of 2-mono-(2-carboxymethylhexyl) trimellitate (2cx-2-MMHTM) and 1-mono-(2-carboxymethylhexyl) trimellitate (2cx-1-MMHTM) was covered by own resources. The sponsor was not involved in the study design as well as in evaluation, interpretation, and publication of the results.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Höllerer, C., Becker, G., Göen, T. et al. Human metabolism and kinetics of tri-(2-ethylhexyl) trimellitate (TEHTM) after oral administration. Arch Toxicol 92, 2793–2807 (2018). https://doi.org/10.1007/s00204-018-2264-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-018-2264-2