Abstract
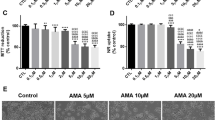
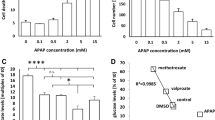
BAL30072 is a new monocyclic β-lactam antibiotic under development which provides a therapeutic option for the treatment of severe infections caused by multi-drug-resistant Gram-negative bacteria. Despite the absence of liver toxicity in preclinical studies in rats and marmosets and in single dose clinical studies in humans, increased transaminase activities were observed in healthy subjects in multiple-dose clinical studies. We, therefore, initiated a comprehensive program to find out the mechanisms leading to hepatocellular injury using HepG2 cells (human hepatocellular carcinoma cell line), HepaRG cells (inducible hepatocytes derived from a human hepatic progenitor cell line), and human liver microtissue preparations. Our investigations demonstrated a concentration- and time-dependent reduction of the ATP content of BAL30072-treated HepG2 cells and liver microtissues. BAL30072 impaired oxygen consumption by HepG2 cells at clinically relevant concentrations, inhibited complexes II and III of the mitochondrial electron transport chain, increased the production of reactive oxygen species (ROS), and reduced the mitochondrial membrane potential. Furthermore, BAL 30072 impaired mitochondrial fatty acid metabolism, inhibited glycolysis, and was associated with hepatocyte apoptosis. Co-administration of N-acetyl-l-cysteine partially protected hepatocytes from BAL30072-mediated toxicity, underscoring the role of oxidative damage in the observed hepatocellular toxicity. In conclusion, BAL30072 is toxic for liver mitochondria and inhibits glycolysis at clinically relevant concentrations. Impaired hepatic mitochondrial function and inhibition of glycolysis can explain liver injury observed in human subjects receiving long-term treatment with this compound.








Similar content being viewed by others
Abbreviations
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- BSO:
-
Buthionine sulphoximine
- CYP:
-
Cytochrome P450
- DILI:
-
Drug-induced liver injury
- DMSO:
-
Dimethylsulfoxide
- DPBS:
-
Dulbecco’s phosphate buffered saline
- ECAR:
-
Extracellular acidification rate
- FCCP:
-
Carbonyl cyanide-4-(trifluoromethoxy)phenylhydrazone
- FDA:
-
Food and drug administration (US)
- GAPDH:
-
Glyceraldehyde 3-phosphate dehydrogenase
- GSH:
-
Reduced glutathione
- H and E:
-
Hematoxylin and Eosin
- HEPES:
-
4-(2-Hydroxyethyl)piperazine-1-ethanesulfonic acid
- HMGB1:
-
High-mobility group protein B1
- LDH:
-
Lactate dehydrogenase
- LPS:
-
Lipopolysaccharide
- NAC:
-
N-acetyl-l-cysteine
- OCR:
-
Oxygen consumption rate
- PBS:
-
Phosphate-buffered saline
- ROS:
-
Reactive oxygen species
- ULN:
-
Upper limit of normal
References
Aithal GP, Watkins PB, Andrade RJ et al (2011) Case definition and phenotype standardization in drug-induced liver injury. Clin Pharmacol Ther 89(6):806–815. doi:10.1038/clpt.2011.58
Anderson ME (1997) Glutathione and glutathione delivery compounds. Adv Pharmacol 38:65–78
Aninat C, Piton A, Glaise D et al (2006) Expression of cytochromes P450, conjugating enzymes and nuclear receptors in human hepatoma HepaRG cells. Drug Metab Dispos 34(1):75–83. doi:10.1124/dmd.105.006759
Bala S, Petrasek J, Mundkur S et al (2012) Circulating microRNAs in exosomes indicate hepatocyte injury and inflammation in alcoholic, drug-induced, and inflammatory liver diseases. Hepatology 56(5):1946–1957. doi:10.1002/hep.25873
Berger B, Donzelli M, Maseneni S et al (2016) Comparison of liver cell models using the basel phenotyping cocktail. Front Pharmacol 7:443. doi:10.3389/fphar.2016.00443
Bouitbir J, Charles AL, Echaniz-Laguna A et al (2012) Opposite effects of statins on mitochondria of cardiac and skeletal muscles: a ‘mitohormesis’ mechanism involving reactive oxygen species and PGC-1. Eur Heart J 33(11):1397–1407. doi:10.1093/eurheartj/ehr224
Chandel NS, Budinger GR (2013) The good and the bad of antibiotics. Sci Transl Med 5(192):192fs25. doi:10.1126/scitranslmed.3006567
Charlot JF, Pretet JL, Haughey C, Mougin C (2004) Mitochondrial translocation of p53 and mitochondrial membrane potential (Delta Psi m) dissipation are early events in staurosporine-induced apoptosis of wild type and mutated p53 epithelial cells. Apoptosis 9(3):333–343
Cui J, Chen Y, Wang HY, Wang RF (2014) Mechanisms and pathways of innate immune activation and regulation in health and cancer. Hum Vaccin Immunother 10(11):3270–3285. doi:10.4161/21645515.2014.979640
Donato MT, Martinez-Romero A, Jimenez N et al (2009) Cytometric analysis for drug-induced steatosis in HepG2 cells. Chem Biol Interact 181(3):417–423. doi:10.1016/j.cbi.2009.07.019
Drose S, Brandt U (2012) Molecular mechanisms of superoxide production by the mitochondrial respiratory chain. Adv Exp Med Biol 748:145–169. doi:10.1007/978-1-4614-3573-0_6
Duewelhenke N, Krut O, Eysel P (2007) Influence on mitochondria and cytotoxicity of different antibiotics administered in high concentrations on primary human osteoblasts and cell lines. Antimicrob Agents Chemother 51(1):54–63. doi:10.1128/AAC.00729-05
Fattinger K, Funk C, Pantze M et al (2001) The endothelin antagonist bosentan inhibits the canalicular bile salt export pump: a potential mechanism for hepatic adverse reactions. Clin Pharmacol Ther 69(4):223–231. doi:10.1067/mcp.2001.114667
Felser A, Blum K, Lindinger PW, Bouitbir J, Krahenbuhl S (2013) Mechanisms of hepatocellular toxicity associated with dronedarone—a comparison to amiodarone. Toxicological sciences: an official journal of the Society of Toxicology 131(2):480–490. doi:10.1093/toxsci/kfs298
Kalghatgi S, Spina CS, Costello JC et al (2013) Bactericidal antibiotics induce mitochondrial dysfunction and oxidative damage in Mammalian cells. Sci Transl Med 5(192):192ra85. doi:10.1126/scitranslmed.3006055
Kaplowitz N (2005) Idiosyncratic drug hepatotoxicity. Nat Rev Drug Discov 4(6):489–499. doi:10.1038/nrd1750
Kaufmann P, Torok M, Hanni A, Roberts P, Gasser R, Krahenbuhl S (2005) Mechanisms of benzarone and benzbromarone-induced hepatic toxicity. Hepatology 41(4):925–935. doi:10.1002/hep.20634
Krahenbuhl S, Talos C, Reichen J (1994) Mechanisms of impaired hepatic fatty acid metabolism in rats with long-term bile duct ligation. Hepatology 19(5):1272–1281
Kroh EM, Parkin RK, Mitchell PS, Tewari M (2010) Analysis of circulating microRNA biomarkers in plasma and serum using quantitative reverse transcription-PCR (qRT-PCR). Methods 50(4):298–301. doi:10.1016/j.ymeth.2010.01.032
Lee WM (2003) Drug-induced hepatotoxicity. N Engl J Med 349(5):474–485. doi:10.1056/NEJMra021844
Leitner JM, Graninger W, Thalhammer F (2010) Hepatotoxicity of antibacterials: pathomechanisms and clinical. Infection 38(1):3–11. doi:10.1007/s15010-009-9179-z
Lim JL, Wilhelmus MM, de Vries HE, Drukarch B, Hoozemans JJ, van Horssen J (2014) Antioxidative defense mechanisms controlled by Nrf2: state-of-the-art and clinical perspectives in neurodegenerative diseases. Arch Toxicol 88(10):1773–1786. doi:10.1007/s00204-014-1338-z
Marchetti P, Castedo M, Susin SA et al (1996) Mitochondrial permeability transition is a central coordinating event of apoptosis. J Exp Med 184(3):1155–1160
Messner S, Agarkova I, Moritz W, Kelm JM (2013) Multi-cell type human liver microtissues for hepatotoxicity testing. Arch Toxicol 87(1):209–213. doi:10.1007/s00204-012-0968-2
Nishikawa T, Bellance N, Damm A et al (2014) A switch in the source of ATP production and a loss in capacity to perform glycolysis are hallmarks of hepatocyte failure in advance liver disease. J Hepatol 60(6):1203–1211. doi:10.1016/j.jhep.2014.02.014
Page MG, Dantier C, Desarbre E (2010) In vitro properties of BAL30072, a novel siderophore sulfactam with activity against multiresistant Gram-negative bacilli. Antimicrob Agents Chemother 54(6):2291–2302. doi:10.1128/AAC.01525-09
Pesta D, Gnaiger E (2012) High-resolution respirometry: oXPHOS protocols for human cells and permeabilized fibers from small biopsies of human muscle. Methods Mol Biol 810:25–58. doi:10.1007/978-1-61779-382-0_3
Reuben A (2004) Hy’s law. Hepatology 39(2):574–578. doi:10.1002/hep.20081
Rial E, Rodriguez-Sanchez L, Gallardo-Vara E, Zaragoza P, Moyano E, Gonzalez-Barroso MM (2010) Lipotoxicity, fatty acid uncoupling and mitochondrial carrier function. Biochim Biophys Acta 1797(6–7):800–806. doi:10.1016/j.bbabio.2010.04.001
Taegtmeyer AB, Haschke M, Tchambaz L et al (2014) A study of the relationship between serum bile acids and propranolol pharmacokinetics and pharmacodynamics in patients with liver cirrhosis and in healthy controls. PLoS One 9(6):e97885. doi:10.1371/journal.pone.0097885
Tailor A, Faulkner L, Naisbitt DJ, Park BK (2015) The chemical, genetic and immunological basis of idiosyncratic drug-induced liver injury. Hum Exp Toxicol 34(12):1310–1317. doi:10.1177/0960327115606529
Temple RJ, Himmel MH (2002) Safety of newly approved drugs: implications for prescribing. JAMA 287(17):2273–2275
Tukov FF, Luyendyk JP, Ganey PE, Roth RA (2007) The role of tumor necrosis factor alpha in lipopolysaccharide/ranitidine-induced inflammatory liver injury. Toxicol Sci 100(1):267–280. doi:10.1093/toxsci/kfm209
Yang H, Antoine DJ, Andersson U, Tracey KJ (2013) The many faces of HMGB1: molecular structure-functional activity in inflammation, apoptosis, and chemotaxis. J Leukoc Biol 93(6):865–873. doi:10.1189/jlb.1212662
Yang X, Yao H, Chen Y et al (2015) Inhibition of Glutathione production induces macrophage CD36 expression and enhances cellular-oxidized low density lipoprotein (oxLDL) uptake. J Biol Chem 290(36):21788–21799. doi:10.1074/jbc.M115.654582
Yilmaz Y (2009) Systematic review: caspase-cleaved fragments of cytokeratin 18—the promises and challenges of a biomarker for chronic liver disease. Aliment Pharmacol Ther 30(11–12):1103–1109. doi:10.1111/j.1365-2036.2009.04148.x
Zamzami N, Marchetti P, Castedo M et al (1995) Reduction in mitochondrial potential constitutes an early irreversible step of programmed lymphocyte death in vivo. J Exp Med 181(5):1661–1672
Zamzami N, Marchetti P, Castedo M et al (1996a) Inhibitors of permeability transition interfere with the disruption of the mitochondrial transmembrane potential during apoptosis. FEBS Lett 384(1):53–57
Zamzami N, Susin SA, Marchetti P et al (1996b) Mitochondrial control of nuclear apoptosis. J Exp Med 183(4):1533–1544
Zhang J, Nuebel E, Wisidagama DR et al (2012) Measuring energy metabolism in cultured cells, including human pluripotent stem cells and differentiated cells. Nat Protoc 7(6):1068–1085. doi:10.1038/nprot.2012.048
Acknowledgements
Scott Eaddy (UNC Institute for Drug Safety Sciences) provided laboratory assistance for quantification of miRNA122 and Total HMGB1 in clinical samples. The authors acknowledge the provision of medical writing services by David Main, Basilea Pharmaceutica International Ltd.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
SK was a member of the Data and Safety Monitoring Board (DSMB) for the phase 1 studies of BAL30072. FP has acted as a consultant for Basilea Pharmaceutica International Ltd.
Financial support
SK was supported by a grant of the Swiss National Science Foundation (31003A_156270).
The studies investigating the potential of BAL30072 to form reactive metabolites that conjugate covalently to proteins were funded with Federal funds from the Department of Health and Human Services; Office of the Assistant Secretary for Preparedness and Response; Biomedical Advanced Research and Development Authority, under Contract No. HHSO100201300010C.
Ethical conduct of clinical studies
The clinical studies of BAL30072 referred to in this manuscript were conducted in accordance with the International Conference on Harmonisation Guideline for Good Clinical Practice E6, with ethical principles consistent with those laid down in the Declaration of Helsinki, and with applicable local laws and regulations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Paech, F., Messner, S., Spickermann, J. et al. Mechanisms of hepatotoxicity associated with the monocyclic β-lactam antibiotic BAL30072. Arch Toxicol 91, 3647–3662 (2017). https://doi.org/10.1007/s00204-017-1994-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-017-1994-x