Abstract
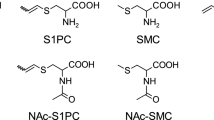
Acrylamide is classified as a probable carcinogen to humans and generated from Maillard reaction. Currently, the short-term exposure to acrylamide was evaluated via external diet sources in vitro or two main mercapturic acid metabolites: N-acetyl-S-(2-carbamoylethyl)-l-cysteine (AAMA) and N-acetyl-S-(2-carbamoyl-2-hydroxyethyl)-l-cysteine (GAMA) in vivo. In the present work, we comprehensively profiled four mercapturic acid metabolites and evaluated their internal exposure in rats and Chinese adolescents. The cumulative excretion of mercapturic acid metabolites contributes 38.4–73.0 and 43.8–63.6 % of total in vivo metabolites of acrylamide in male and female rats, respectively, when 1, 10, and 50 mg/kg bw of acrylamide were orally administered. Toxicokinetic study revealed that the conversion of acrylamide into glycidamide and glutathione coupling process is highly related to the gender and oral gavage dose via evaluating kinetic parameters, accumulative excretion percentages, and molar ratios of oxidative to reductive metabolism. In human study, a total of 101 Chinese adolescents (41 men and 60 women) were enrolled and served with a meal of potato chips, corresponding to a single-dose (12.6 μg/kg bw) exposure to acrylamide. Toxicokinetic work showed that AAMA is an early and predominant metabolite appearing as a biomarker in urine. N-acetyl-S-(2-carbamoylethyl)-l-cysteine-sulfoxide (AAMA-sul), an oxidative product from AAMA, exhibits a higher peak concentration than GAMA and N-acetyl-S-(1-carbamoyl-2-hydroxyethyl)-l-cysteine (iso-GAMA) during the whole 48-h toxicokinetic period. The internal exposure via four mercapturic acid metabolites is associated with the gender and body mass index characteristics. Thus, current study aims at mercapturic acid metabolites as urinary biomarkers and provides comprehensive insights into the short-term internal exposure to acrylamide.


Similar content being viewed by others
References
Bjellaas T, Ølstørn HB, Becher G, Alexander J, Knutsen SH, Paulsen JE (2007) Urinary metabolites as biomarkers of acrylamide exposure in mice following dietary crisp bread administration or subcutaneous injection. Toxicol Sci 100:374–380
Boettcher MI, Bolt HM, Drexler H, Angerer J (2006) Excretion of mercapturic acids of acrylamide and glycidamide in human urine after single oral administration of deuterium-labelled acrylamide. Arch Toxicol 80:55–61
Bongers ML, Hogervorst JG, Schouten LJ, Goldbohm RA, Schouten HC, van den Brandt PA (2012) Dietary acrylamide intake and the risk of lymphatic malignancies: the Netherlands Cohort Study on diet and cancer. PLoS One 7:e38016
Bonsnes RW, Taussky HH (1945) On the colorimetric determination of creatinine by the Jaffe reaction. J Biol Chem 158:581–591
Doerge DR, Twaddle NC, Boettcher MI, McDaniel LP, Angerer J (2007) Urinary excretion of acrylamide and metabolites in Fischer 344 rats and B6C3F1 mice administered a single dose of acrylamide. Toxicol Lett 169:34–42
Exon JH (2006) A review of the toxicology of acrylamide. J Toxicol Environ Health B Crit Rev 9:397–412
Fennell TR, Sumner SC, Snyder RW, Burgess J, Friedman MA (2006) Kinetics of elimination of urinary metabolites of acrylamide in humans. Toxicol Sci 93:256–267
Freisling H, Moskal A, Ferrari P, Nicolas G, Knaze V, Clavel-Chapelon F, Boutron-Ruault MC, Nailler L, Teucher B, Grote VA, Boeing H, Clemens M, Tjønneland A, Olsen A, Overvad K, Quirós JR, Duell EJ, Sánchez MJ, Amiano P, Chirlaque MD, Barricarte A, Khaw KT, Wareham NJ, Crowe FL, Gallo V, Oikonomou E, Naska A, Trichopoulou A, Palli D, Agnoli C, Tumino R, Polidoro S, Mattiello A, Bueno-de-Mesquita HB, Ocké MC, Peeters PH, Wirfält E, Ericson U, Bergdahl IA, Johansson I, Hjartåker A, Engeset D, Skeie G, Riboli E, Slimani N (2013) Dietary acrylamide intake of adults in the European Prospective Investigation into Cancer and Nutrition differs greatly according to geographical region. Eur J Nutr 52:1369–1380
Friedman M (2015) Acrylamide: inhibition of formation in processed food and mitigation of toxicity in cells, animals, and humans. Food Funct 6:1752–1772
Fuhr U, Boettcher MI, Kinzig-Schippers M, Weyer A, Jetter A, Lazar A, Taubert D, Tomalik-Scharte D, Pournara P, Jakob V, Harlfinger S, Klaassen T, Berkessel A, Angerer J, Sörgel F, Schömig E (2006) Toxicokinetics of acrylamide in humans after ingestion of a defined dose in a test meal to improve risk assessment for acrylamide carcinogenicity. Cancer Epidemiol Biomark Prev 15:266–271
Hartmann EC, Boettcher MI, Bolt HM, Drexler H, Angerer J (2009) N-Acetyl-S-(1-carbamoyl-2-hydroxy-ethyl)-l-cysteine (iso-GAMA) a further product of human metabolism of acrylamide: comparison with the simultaneously excreted other mercaptuic acids. Arch Toxicol 83:731–734
Hirvonen T, Jestoi M, Tapanainen H, Valsta L, Virtanen SM, Sinkko H, Kronberg-Kippilä C, Kontto J, Virtamo J, Simell O, Peltonen K (2011) Dietary acrylamide exposure among Finnish adults and children: the potential effect of reduction measures. Food Addit Contam A Chem 28:1483–1491
Hogervorst JG, Schouten LJ, Konings EJ, Goldbohm RA, van den Brandt PA (2008) Dietary acrylamide intake and the risk of renal cell, bladder, and prostate cancer. Am J Clin Nutr 87:1428–1438
Hogervorst JGF, Baars BJ, Schouten LJ, Konings EJ, Goldbohm RA, van den Brandt PA (2010) The carcinogenicity of dietary acrylamide intake: a comparative discussion of epidemiological and experimental animal research. Crit Rev Toxicol 40:485–512
Ilyin SE, Belkowski SM, Plata-Salaman CR (2004) Biomarker discovery and validation: technologies and integrative approaches. Trends Biotechnol 22:411–416
International Agency for Research on Cancer (IARC) (1994) Summary of data reported and evaluation: acrylamide. http://monographs.iarc.fr/ENG/Monographs/ vol60/volume60.pdf
Irving RM, Pinkerton ME, Elfarra AA (2013) Characterization of the chemical reactivity and nephrotoxicity of N-acetyl-S-(1,2-dichlorovinyl)-l-cysteine sulfoxide, a potential reactive metabolite of trichloroethylene. Toxicol Appl Pharmacol 267:1–10
Je Y (2015) Dietary acrylamide intake and risk of endometrial cancer in prospective cohort studies. Arch Gynecol Obstet 291:1395–1401
Kadry AM, Friedman MA, Abdel-Rahman MS (1999) Pharmacokinetics of acrylamide after oral administration in male rats. Environ Toxicol Pharmacol 7:127–133
Kopp EK, Dekant W (2009) Toxicokinetics of acrylamide in rats and humans following single oral administration of low doses. Toxicol Appl Pharmacol 235:135–142
Lee JH, Lee KJ, Ahn R, Kang HS (2014) Urinary concentrations of acrylamide (AA) and N-acetyl-S-(2-carbamoylethyl)-cysteine (AAMA) and associations with demographic factors in the South Korean population. Int J Hyg Environ Health 217:751–757
Li D, Wang P, Liu Y, Hu X, Chen F (2016) Metabolism of acrylamide: interindividual and interspecies differences as well as the application as biomarkers. Curr Drug Metab 17:317–326
Lin Y, Lagergren J, Lu Y (2011) Dietary acrylamide intake and risk of esophageal cancer in a population-based case-control study in Sweden. Int J Cancer 128:676–681
Lineback DR, Coughlin JR, Stadler RH (2012) Acrylamide in foods: a review of the science and future considerations. Annu Rev Food Sci Technol 3:15–35
Luo YS, Long TY, Shen LC, Huang SL, Chiang SY, Wu KY (2015) Synthesis, characterization and analysis of the acrylamide- and glycidamide-glutathione conjugates. Chem Biol Interact 237:38–46
Maronpot RR, Thoolen RJMM, Hansen B (2015) Two-year carcinogenicity study of acrylamide in Wistar Han rats with in utero exposure. Exp Toxicol Pathol 67:189–195
Mojska H, Gielecińska I, Zielińska A, Winiarek J, Sawicki W (2016) Estimation of exposure to dietary acrylamide based on mercapturic acids level in urine of Polish women post partum and an assessment of health risk. J Expo Sci Environ Epidemiol 26:288–295
Mottram DS, Wedzicha BL, Dodson AT (2002) Acrylamide is formed in the Maillard reaction. Nature 419:448–449
Obón-Santacana M, Lujan-Barroso L, Freisling H, Cadeau C, Fagherazzi G, Boutron-Ruault MC, Kaaks R, Fortner RT, Boeing H, Ramón Quirós J, Molina-Montes E, Chamosa S, Castaño JM, Ardanaz E, Khaw KT, Wareham N, Key T, Trichopoulou A, Lagiou P, Naska A, Palli D, Grioni S, Tumino R, Vineis P, De Magistris MS, Bueno-de-Mesquita HB, Peeters PH, Wennberg M, Bergdahl IA, Vesper H, Riboli E, Duell EJ (2016) Dietary and lifestyle determinants of acrylamide and glycidamide hemoglobin adducts in non-smoking postmenopausal women from the EPIC cohort. Eur J Nutr. doi:10.1007/s00394-016-1165-5
Pelucchi C, La Vecchia C, Bosetti C, Boyle P, Boffetta P (2011) Exposure to acrylamide and human cancer—a review and meta-analysis of epidemiologic studies. Ann Oncol 22:1487–1499
Ramu K, Fraiser LH, Mamiya B, Ahmed T, Kehrer JP (1995) Acrolein mercapturates: synthesis, characterization, and assessment of their role in the bladder toxicity of cyclophosphamide. Chem Res Toxicol 8:515–524
Ruenz M, Bakuradze T, Eisenbrand G, Richling E (2016) Monitoring urinary mercapturic acids as biomarkers of human dietary exposure to acrylamide in combination with acrylamide uptake assessment based on duplicate diets. Arch Toxicol 90:873–881
Stadler RH, Blank I, Varga N, Robert F, Hau J, Guy PA, Robert MC, Riediker S (2002) Acrylamide from Maillard reaction products. Nature 419:449–450
Tareke E, Rydberg P, Karlsson P, Eriksson S, Törnqvist M (2002) Analysis of acrylamide, a carcinogen formed in heated foodstuffs. J Agric Food Chem 50:4998–5006
Vesper HW, Caudill SP, Osterloh JD, Meyers T, Scott D, Myers GL (2010) Exposure of the U.S. population to acrylamide in the National Health and Nutrition Examination Survey 2003–2004. Environ Health Perspect 118:278–283
Watzek N, Scherbl D, Feld J, Berger F, Doroshyenko O, Fuhr U, Tomalik-Scharte D, Baum M, Eisenbrand G, Richling E (2012) Profiling of mercapturic acids of acrolein and acrylamide in human urine after consumption of potato crisps. Mol Nutr Food Res 56:1825–1837
Watzek N, Scherbl D, Schug M, Hengstler JG, Baum M, Habermeyer M, Richling E, Eisenbrand G (2013) Toxicokinetics of acrylamide in primary rat hepatocytes: coupling to glutathione is faster than conversion to glycidamide. Arch Toxicol 87:1545–1556
Wilson KM, Mucci LA, Rosner BA, Willett WC (2010) A prospective study on dietary acrylamide intake and the risk for breast, endometrial, and ovarian cancers. Cancer Epidemiol Biomark Prev 19:2503–2515
Wilson KM, Giovannucci E, Stampfer MJ, Mucci LA (2012) Dietary acrylamide and risk of prostate cancer. Int J Cancer 131:479–487
World Health Organization Expert Consultation (2004) Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 363:157–163
Xu Y, Cui B, Ran R, Liu Y, Chen H, Kai G, Shi J (2014) Risk assessment, formation, and mitigation of dietary acrylamide: current status and future prospects. Food Chem Toxicol 69:1–12
Zhang Y, Jiao JJ, Cai ZX, Zhang Y, Ren YP (2007) An improved method validation for rapid determination of acrylamide in foods by ultra-performance liquid chromatography combined with tandem mass spectrometry. J Chromatogr A 1142:194–198
Zhou PP, Zhao YF, Liu HL, Ma YJ, Li XW, Yang X, Wu YN (2013) Dietary exposure of the Chinese population to acrylamide. Biomed Environ Sci 26:421–429
Acknowledgments
We gratefully acknowledge the financial support from National Natural Science Foundation of China (Grant No. 21277123) and the Foundation for the Author of Nationally Excellent Doctoral Dissertation of China (Grant No. 201260).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, Q., Chen, X., Ren, Y. et al. Toxicokinetics and internal exposure of acrylamide: new insight into comprehensively profiling mercapturic acid metabolites as short-term biomarkers in rats and Chinese adolescents. Arch Toxicol 91, 2107–2118 (2017). https://doi.org/10.1007/s00204-016-1869-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-016-1869-6